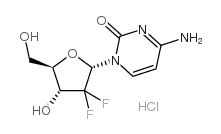
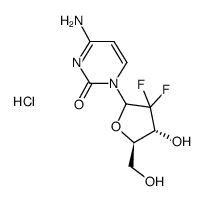
122111-03-9
| 中文名 | 盐酸吉西他滨 |
|---|---|
| 英文名 | gemcitabine hydrochloride |
| 中文别名 |
盐酸吉西他宾
4-氨基-1-(3,3-二氟-4-羟基-5-羟甲基四氢呋喃-2-基)-1H-嘧啶-2-酮盐酸盐 |
| 英文别名 |
dFdC,Gemzar,Lilly)
MFCD01735988 dFdC Gemzar (Lilly) LY-188011 dFdC dFdCyd 4-Amino-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one hydrochloride Gemcitabine hydrochloride 2'-Deoxy-2',2'-difluorocytidine 4-Amino-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-2(1H)-pyrimidinone hydrochloride Cytidine, 2'-deoxy-2',2'-difluoro-, hydrochloride (1:1) 2'-Deoxy-2',2'-difluorocytidine hydrochloride (1:1) Gemcitabine HCl Gemcitabine HCl(TABINES) Gemcitabine (Hydrochloride) |
| 描述 | Gemcitabine hydrochloride是一种DNA合成抑制剂,在131.4 nM -2,PANC-1,PL-45和AsPC-1细胞中的 IC50 分别为37.6,42.9,92.7,89.3 和 131.4 nM。 |
|---|---|
| 相关类别 | |
| 靶点 |
DNA synthesis[1] |
| 体外研究 | MTS测定证明,15nM的吉西他滨,50μM的吲哚-3-甲醇(I3C)和该组合不影响hTERT-HPNE细胞活力。然而,用15nM的吉西他滨,50μM的I3C和该组合治疗分别导致BxPC-3细胞31%,19%和72%的细胞死亡[1]。 |
| 体内研究 | 研究的目的是配制盐酸吉西他滨(盐酸吉西他滨)的PLGA纳米颗粒(NPs),以通过Peyer's贴剂的M细胞吸收来增强口服生物利用度。由于半衰期短(8-17分钟),快速代谢和有限的肿瘤摄取,盐酸吉西他滨可作为静脉输注使用。与口服给药后的普通药物溶液相比,装载吉西他滨的PLGA NPs的相对生物利用度提高了21.47倍[2]。静脉注射剂量为50,100和120,以及300 mg/kg的吉西他滨后,最高剂量导致相当大的体重减轻(从第一次注射的第3天开始评估的所有时间点p <0.05)未治疗组的死亡率和完全死亡率,而120 mg/kg被确定为致死剂量10%(LD10)和100 mg/kg被认为是最大耐受剂量,不会导致任何死亡和最小体重减轻[3]。 |
| 细胞实验 | 将细胞(人胰腺细胞系,Mia PaCa-2,BxPC-3,AsPC-1,PANC-1,PL-45和正常胰腺导管上皮细胞,hTERT-HPNE细胞)接种到96孔板中(3000细胞/孔)一式三份。孵育过夜后,更换培养基并用I3C和/或NBMPR处理细胞24小时。再次更换培养基,并在存在或不存在相同浓度的I3C和/或NBMPR的情况下,在含有不同浓度的吉西他滨的培养基中培养细胞48小时。然后将细胞进行CellTiter 96 AQueous One Solution Cell Proliferation Assay(MTS)。在加入20μLMTS试剂/孔后2小时测量490nm处的吸光度[1]。载有盐酸吉西他滨的NPs对Caco-2细胞的体外细胞毒性进行6小时以检查NPs在转运/渗透性研究期间的毒性,并且使用MTT测定法评估对K562细胞的抗增殖作用48小时。将细胞在96孔板中以1.0×10 4个细胞/孔的接种密度培养48小时。通过在CO 2培养箱中在37℃下用150μL样品溶液(0.1,1,10,100μg/ mL)替换每个孔中的培养基来开始实验。孵育4,24和48小时后,除去培养基并向每个孔中加入150μL无血清培养基中的MTT试剂(1mg / mL)。然后将板在37℃下再孵育4小时。在孵育期结束时,除去培养基并用150μLDMSO溶解细胞内甲,,并通过在具有Soft Max Pro的微板多检测仪器SpectraMax M2上读取590nm处的吸光度来定量。培养基处理的细胞用作对照。基于相对于暴露于阴性对照的细胞的吸光度测量的吸光度计算细胞活力百分比[2]。 |
| 动物实验 | 大鼠[2]对三组雄性Wistar大鼠(n = 6)进行单次口服剂量生物利用度研究。借助注射器和婴儿喂食管口服给予制剂。第一组给予蒸馏水,第二组给予盐酸吉西他滨在蒸馏水中的溶液,第三组给予加有盐酸吉西他滨的NPs,剂量为10mg / kg。在口服剂量后0.5,1,2,4,24,48,72小时,借助毛细管通过眶后静脉丛穿刺抽取血样(0.3mL)。将样品收集在含有10μM四氢尿苷的肝素化Eppendorf管中,以3400rpm离心15分钟并收集血浆。向其中加入200μL乙腈并涡旋5分钟,然后以5000rpm离心15分钟。分离有机相,并在真空烘箱中减压蒸发。将残余物溶于流动相(0.15mL)中,涡旋1分钟,然后以13,000rpm离心5分钟。然后将20μL上清液注入HPLC柱中并通过HPLC分析。将重约15至18g的小鼠[3] DBA / 2小鼠(5-6周龄)用于该研究。给小鼠随意提供标准小鼠食物和水。在体外维持L1210 wt白血病细胞,并将它们静脉内(1×105)注射到小鼠体内,以形成全身性转移性白血病模型。将小鼠分成6组,每组7至8只小鼠:未处理,用角鲨烯纳米颗粒处理,用100mg / kg吉西他滨处理,用吉西他滨20mg / kg等效SQgem纳米组装物处理,用100mg / kg阿糖胞苷处理,和每天用100mg / kg阿糖胞苷治疗5天。在注射白血病细胞(第0天)后,所有组的小鼠在第1,5,9和14天(即注射白血病细胞后数天)通过静脉注射接受治疗,除了未治疗组和组。每天用静脉注射途径用阿糖胞苷治疗。定期监测小鼠的体重差异和存活率。 |
| 参考文献 |
| 沸点 | 482.7ºC at 760 mmHg |
|---|---|
| 熔点 | >250°C dec. |
| 分子式 | C9H12ClF2N3O4 |
| 分子量 | 299.659 |
| 精确质量 | 299.048431 |
| PSA | 110.60000 |
| LogP | 0.09460 |
| 外观性状 | 固体;White to Almost white powder to crystal |
| 蒸汽压 | 2.41E-11mmHg at 25°C |
| 储存条件 | Desiccate at +4°C |
| 计算化学 | 1、 疏水参数计算参考值(XlogP): 2、 氢键供体数量:4 3、 氢键受体数量:7 4、 可旋转化学键数量:2 5、 互变异构体数量:3 6、 拓扑分子极性表面积(TPSA):108 7、 重原子数量:19 8、 表面电荷:0 9、 复杂度:426 10、同位素原子数量:0 11、确定原子立构中心数量:3 12、不确定原子立构中心数量:0 13、确定化学键立构中心数量:0 14、不确定化学键立构中心数量:0 15、共价键单元数量:2 |
| 更多 | 1. 性状:未确定 2. 密度(g/mL,20℃):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):>250 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,KPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(Pa,20ºC):未确定 12. 饱和蒸气压(KPa,20ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:未确定 |
|
Section I.Chemical Product and Company Identification Chemical Name Gemcitabine Hydrochloride Portland OR SynonymCytidine, 2'-deoxy-2',2'-difluoro-, hydrochloride (1:1) (CA INDEX NAME) Chemical FormulaC9H11F2N3O4 · HCl CAS Number122111-03-9
Section II.Composition and Information on Ingredients Toxicology Data Chemical NameCAS Number Percent (%)TLV/PEL Min. 98.0 Not available.Rat LD50 (intravenous) 236 mg/kg Gemcitabine Hydrochloride122111-03-9 (HPLC,N)Mouse LD50 (intravenous) 500 mg/kg Section III. Hazards Identification Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the Acute Health Effects eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound. CARCINOGENIC EFFECTS : Not available. Chronic Health Effects MUTAGENIC EFFECTS : Not available. TERATOGENIC EFFECTS : Not available. DEVELOPMENTAL TOXICITY: Reproductive effects. Mouse TDLo Intravenous 15 mg/kg, female 6-15 days of pregnancy Toxic Effects: Maternal Effects - Parturition Effects on Fertility - Post-implantation mortality Effects on Fertility - Litter size Mouse TDLo Intravenous 15 mg/kg, female 6-15 days of pregnancy Toxic Effects: Maternal Effects - Other effects Effects on Embryo or Fetus - Fetotoxicity Effects on Embryo or Fetus - Fetal death Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions. Section IV.First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention. Skin ContactIn case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or Inhalation waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not improve. INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat. Ingestion Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material was ingested; the absence of such signs, however, is not conclusive. Section V.Fire and Explosion Data Not available. May be combustible at high temperature.Auto-Ignition Flammability Flash PointsFlammable LimitsNot available. Not available. These products are toxic carbon oxides (CO, CO2), nitrogen oxides (NO, NO2), halogenated compounds. Combustion Products WARNING: Highly toxic HCl gas is produced during combustion. WARNING: Highly toxic HF gas is produced during combustion. Fire Hazards Not available. Continued on Next Page Gemcitabine Hydrochloride Explosion HazardsRisks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. Fire Fighting MediaSMALL FIRE: Use DRY chemical powder. LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet. and Instructions Consult with local fire authorities before attempting large scale fire-fighting operations. Section VI.Accidental Release Measures Spill CleanupIrritating material. This material is a reproductive effector. Use a shovel to put the material into a convenient waste disposal container. Consult federal, state, and/or local authorities for Instructions assistance on disposal. Section VII. Handling and Storage IRRITANT. REPRODUCTIVE EFFECTOR. Keep away from heat. Mechanical exhaust required. When not in use, tightly Handling and Storage seal the container and store in a dry, cool place. Avoid excessive heat and light. Do not breathe dust. Information Always store away from incompatible compounds such as oxidizing agents. Section VIII. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSplash goggles. Lab coat. Dust respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent. Not available. Exposure Limits Section IX. Physical and Chemical Properties Physical state @ 20°CSolid. (White crystalline powder.)SolubilitySoluble in hot water. Not available. Specific Gravity Molecular Weight299.66Partition CoefficientNot available. Boiling PointNot available.Not applicable. Vapor Pressure Melting Point287 to 292°C (548.6 to 557.6°F) (dec.)Vapor DensityNot available. Not available.Not available. Refractive IndexVolatility Critical TemperatureNot available.OdorNot available. Not available.Not available. ViscosityTaste Section X.Stability and Reactivity Data This material is stable if stored under proper conditions. (See Section VII for instructions) Stability Conditions of InstabilityAvoid excessive heat and light. IncompatibilitiesReactive with oxidizing agents. Section XI. Toxicological Information RTECS NumberHA3840000 Eye Contact. Ingestion. Inhalation. Routes of Exposure Rat LD50 (intravenous) 236 mg/kg Toxicity Data Mouse LD50 (intravenous) 500 mg/kg CARCINOGENIC EFFECTS : Not available. Chronic Toxic Effects MUTAGENIC EFFECTS : Not available. TERATOGENIC EFFECTS : Not available. DEVELOPMENTAL TOXICITY: Reproductive effects. Mouse TDLo Intravenous 15 mg/kg, female 6-15 days of pregnancy Toxic Effects: Maternal Effects - Parturition Effects on Fertility - Post-implantation mortality Effects on Fertility - Litter size Mouse TDLo Intravenous 15 mg/kg, female 6-15 days of pregnancy Toxic Effects: Maternal Effects - Other effects Effects on Embryo or Fetus - Fetotoxicity Effects on Embryo or Fetus - Fetal death Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions. Continued on Next Page Gemcitabine Hydrochloride Acute Toxic EffectsIrritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound. Section XII.Ecological Information Not available. Ecotoxicity Environmental FateNot available. Section XIII. Disposal Considerations Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a Waste Disposal combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when disposing of the substance. Section XIV. Transport Information DOT ClassificationNot a DOT controlled material (United States). PIN NumberNot applicable. Proper Shipping NameNot applicable. Packing Group (PG)Not applicable. DOT Pictograms Section XV. Other Regulatory Information and Pictograms TSCA Chemical InventoryThis product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40 CFR 720.36 (C) for those products not on the inventory list: (EPA) (i) These products are supplied solely for use in research and development by or under the supervision of a technically qualified individual as defined in 40 CFR 720.0 et sec. (ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be supplied on an MSDS sheet. WHMIS ClassificationNot available. (Canada) EINECS Number (EEC) 601-823-3 EEC Risk StatementsR36/37/38- Irritating to eyes, respiratory system and skin. R60- May impair fertility. R61- May cause harm to the unborn child. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 生殖毒性:受孕6-15天的小鼠静脉注射TDLo:15mg/kgSEX/DURATION; CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 符号 |

GHS08 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H360 |
| 警示性声明 | P201-P280-P308 + P313 |
| 危害码 (欧洲) | Xi: Irritant; |
| 风险声明 (欧洲) | R21 |
| 安全声明 (欧洲) | 25-26-36/37-53 |
| 危险品运输编码 | NONH for all modes of transport |
| 海关编码 | 2934999090 |
|
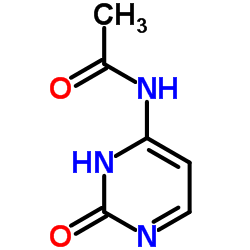
~89% 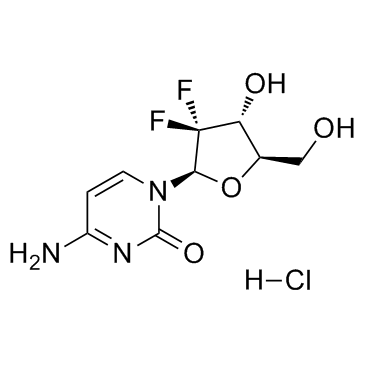
122111-03-9 |
| 文献:Hanmi Pharm. Co.,Ltd Patent: US2007/249818 A1, 2007 ; Location in patent: Page/Page column 10 ; |
|
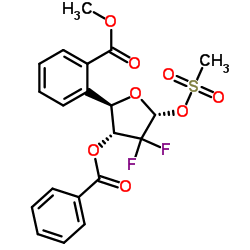
~51% 
122111-03-9 |
| 文献:KANNAN, Vishwanath; KASH, Vishwanathan Patent: WO2010/29574 A2, 2010 ; Location in patent: Page/Page column 8-9 ; |
|
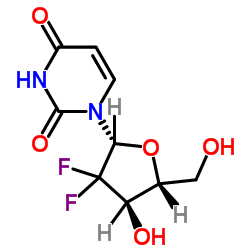
~% 
122111-03-9 |
| 文献:WO2007/117760 A2, ; Page/Page column 21 ; |
|
~% 
122111-03-9 |
| 文献:WO2005/95430 A1, ; Page/Page column 18-20 ; |
|
~% 
122111-03-9 |
| 文献:WO2007/49294 A1, ; Page/Page column 10-11 ; |
|
~% 
122111-03-9 |
| 文献:WO2007/112473 A1, ; Page/Page column 8-9 ; |
| 上游产品 6 | |
|---|---|
| 下游产品 4 | |
| 海关编码 | 2934999090 |
|---|---|
| 中文概述 | 2934999090. 其他杂环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |





![2'-Deoxy-3',5'-bis-O-[dimethyl(2-methyl-2-propanyl)silyl]-2',2'-d ifluorocytidine结构式](https://image.chemsrc.com/caspic/046/688009-09-8.png)