Thiocolchicine
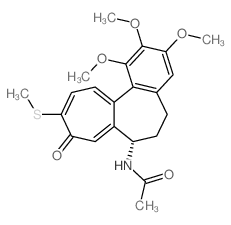
Thiocolchicine结构式

|
常用名 | Thiocolchicine | 英文名 | Thiocolchicine |
|---|---|---|---|---|
| CAS号 | 2730-71-4 | 分子量 | 415.50300 | |
| 密度 | 1.27g/cm3 | 沸点 | 729.1ºC at 760mmHg | |
| 分子式 | C22H25NO5S | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | 394.7ºC | |
| 符号 |



GHS05, GHS06, GHS08 |
信号词 | Danger |
Thiocolchicine用途Thiocolchicine 是秋水仙碱 (HY-16569) 的衍生物,是一种有效的微管蛋白聚合 (tubulin polymerization) 抑制剂(IC50=2.5 µM),其 Ki 值为 0.7 µM 竞争性结合微管蛋白。Thiocolchicine 可以作为 ADC 的毒素分子。 |
| 中文名 | 硫代秋水仙碱 |
|---|---|
| 英文名 | N-[(7S)-1,2,3-trimethoxy-10-methylsulfanyl-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide |
| 英文别名 | 更多 |
| 描述 | Thiocolchicine 是秋水仙碱 (HY-16569) 的衍生物,是一种有效的微管蛋白聚合 (tubulin polymerization) 抑制剂(IC50=2.5 µM),其 Ki 值为 0.7 µM 竞争性结合微管蛋白。Thiocolchicine 可以作为 ADC 的毒素分子。 |
|---|---|
| 相关类别 | |
| 体外研究 | 硫秋水仙碱对MCF-7、LoVo、LoVo/DX、A-549和BALB/3T3细胞的IC50值分别为0.01μM、0.021μM、0.398μM、0.011μM和0.114μM[3]。硫秋水仙碱(1nm-100μM;24-72小时)显示细胞周期阻滞活性与乳腺癌细胞生长抑制之间的关系。它抑制MDA-MB-231和多药耐药(MDR)MCF-7 ADRr乳腺癌细胞的增殖,IC50s分别为0.6nm和400nm,以及MDR CEM-VBL白血病细胞(IC50=50nm)[2]。 |
| 参考文献 |
| 密度 | 1.27g/cm3 |
|---|---|
| 沸点 | 729.1ºC at 760mmHg |
| 分子式 | C22H25NO5S |
| 分子量 | 415.50300 |
| 闪点 | 394.7ºC |
| 精确质量 | 415.14500 |
| PSA | 99.16000 |
| LogP | 3.97580 |
| 外观性状 | 固体 |
| 蒸汽压 | 4.12E-21mmHg at 25°C |
| 折射率 | 1.609 |
| 储存条件 | 2-8°C,密封,干燥 |
| 符号 |



GHS05, GHS06, GHS08 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H300 + H330-H318-H340 |
| 警示性声明 | P201-P260-P264-P280-P284-P301 + P310 |
| 危害码 (欧洲) | T+ |
| 危险品运输编码 | UN 1544PSN1 6.1 / PGI |
| Thiocolchicine上游产品 7 | |
|---|---|
| Thiocolchicine下游产品 7 | |
|
Antiproliferative activity of colchicine analogues on MDR-positive and MDR-negative human cancer cell lines.
Anticancer Drug Des. 13 , 19-33, (1998) In this study the in vitro antitumor activity of a series of 20 colchicine analogues was tested and compared with colchicine and thiocolchicine on three different human cancer cell lines, two of which... |
|
|
Antitumor agents--CLXXV. Anti-tubulin action of (+)-thiocolchicine prepared by partial synthesis.
Bioorg. Med. Chem. 5(12) , 2277-82, (1997) (+)-Thiocolchicine (2b) was prepared from (+/-)-colchicine (1) in a five-step reaction sequence that included chromatographic separation of appropriate camphanylated diastereomers. Acid hydrolysis of ... |
|
|
Thiocolchicine dimers: a novel class of topoisomerase-I inhibitors.
Biochem. Pharmacol. 69(1) , 113-21, (2005) During a cellular screening of thiocolchicine analogs, thiocolchicine dimers resulted particularly active in cisplatin-resistant A2780-CIS cells. In order to discover by which mechanism(s) thiocolchic... |
| thiocolhicine |
| N-[(7S)-1,2,3-trimethoxy-10-methylsulfanyl-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide |
| 10-Demethoxy-10-methylthiocolchicine |
| N-((S)-1,2,3-trimethoxy-10-methylsulfanyl-9-oxo-5,6,7,9-tetrahydro-benzo[a]heptalen-7-yl)-acetamide |
| Thiocolchicine |
| thiocolchicine |
| EINECS 220-346-8 |
| Colchicine,10-thio |
| N-((S)-1,2,3-Trimethoxy-10-methylmercapto-9-oxo-5,6,7,9-tetrahydro-benzo[a]heptalen-7-yl)-acetamid |
| Thiocholchicine |
| Colchicine,10-demethoxy-10-(methylthio) |

 CAS号64-86-8
CAS号64-86-8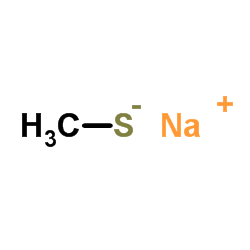 CAS号5188-07-8
CAS号5188-07-8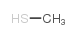 CAS号74-93-1
CAS号74-93-1![Benzo[a]heptalen-9(5H)-one,7-amino-6,7-dihydro-1,2,3-trimethoxy-10-(methylthio)-, (7S)-结构式](https://image.chemsrc.com/caspic/394/2731-16-0.png) CAS号2731-16-0
CAS号2731-16-0 CAS号108-24-7
CAS号108-24-7![N-(1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl)acetamide结构式](https://image.chemsrc.com/caspic/493/209810-38-8.png) CAS号209810-38-8
CAS号209810-38-8 CAS号186581-53-3
CAS号186581-53-3![Acetamide,N-[(7S)-5,6,7,9-tetrahydro-1,2,3-trimethoxy-10-(methylsulfonyl)-9-oxobenzo[a]heptalen-7-yl]-结构式](https://image.chemsrc.com/caspic/095/2826-75-7.png) CAS号2826-75-7
CAS号2826-75-7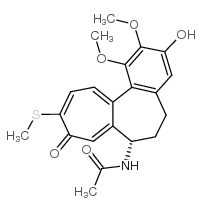 CAS号87424-25-7
CAS号87424-25-7![N-(1,2,3-trimethoxy-10-methylsulfinyl-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl)acetamide结构式](https://image.chemsrc.com/caspic/178/76189-03-2.png) CAS号76189-03-2
CAS号76189-03-2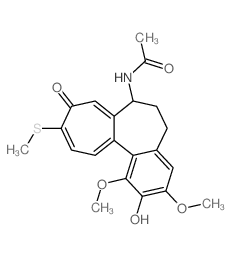 CAS号87424-26-8
CAS号87424-26-8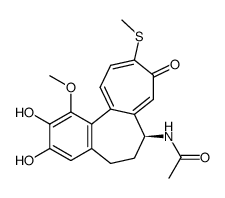 CAS号51296-12-9
CAS号51296-12-9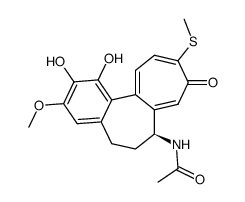 CAS号51296-11-8
CAS号51296-11-8
