Trimethylsilyl trifluoromethanesulfonate
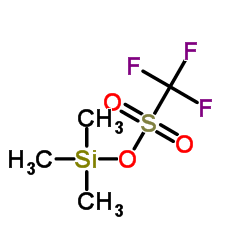
Trimethylsilyl trifluoromethanesulfonate structure
|
Common Name | Trimethylsilyl trifluoromethanesulfonate | ||
|---|---|---|---|---|
| CAS Number | 27607-77-8 | Molecular Weight | 222.258 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 140.0±35.0 °C at 760 mmHg | |
| Molecular Formula | C4H9F3O3SSi | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 38.5±25.9 °C | |
| Symbol |


GHS02, GHS05 |
Signal Word | Danger | |
|
Trimethylsilyl trifluoromethanesulfonate-promoted reductive 2'-O-arylmethylation of ribonucleoside derivatives.
Nucleosides Nucleotides Nucleic Acids 30(6) , 446-56, (2011) Arylmethyl groups such as benzyl, p-methoxybenzyl, and 1-pyrenylmethyl groups were introduced to the 2'-O-position of nucleosides by reductive etherification. Combining corresponding aromatic aldehydes with 2'-O-trimethylsilylnucleoside derivatives in the pre... |
|
|
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) assisted facile deprotection of N,O-acetonides.
J. Org. Chem. 73(2) , 752-5, (2008) Employing TMSOTf as an easily available reagent, we have developed a mild and efficient method for the deprotection of both terminal and internal N,0-acetonide functionalities. Various regularly used protecting groups and common organic functional moieties we... |
|
|
Nucleophilic substitution at the anomeric position of 1,2-O-isopropylidenefuranose derivatives. A novel stereoselective synthesis of cyclic phosphates analogous to cAMP.
Carbohydr. Res. 341(18) , 2883-90, (2006) 1,2-O-Isopropylidenefuranose derivatives were treated with various nucleophiles in the presence of either BF(3).OEt(2) or trimethylsilyl trifluoromethanesulfonate (TMSOTf) leading to substitution products in a regio- and stereoselective manner. In particular,... |
|
|
Intramolecular formal [4 + 2] cycloaddition of nitriles with amides triggered by TMSOTf/Et3N: highly efficient construction of pyrrolo[1,2-a]pyrimidin-4(6H)-ones.
J. Org. Chem. 74(15) , 5699-702, (2009) By treatment with TMSOTf/Et3N, N-(2-cyanoarylmethyl)-substituted acrylamides or beta-ketoamides underwent N-addition cascades under mild conditions to afford the corresponding pyrrolo[1,2-a]pyrimidin-4(6H)-ones as the formal [4 + 2] cycloaddition products in ... |
|
|
Diastereoselective synthesis of substituted dihydropyrans via an oxonium-ene cyclization reaction.
Org. Biomol. Chem. 10(43) , 8730-8, (2012) Substituted dihydropyrans can be efficiently synthesized in good yields with excellent diastereoselectivity from the reaction of aldehydes or epoxides and ethyl 3-alkyl-3-hydroxy-5-methylhex-5-enoate via an oxonium-ene cyclization reaction catalyzed by trimet... |
|
|
Facile synthesis of N-(1-alkenyl) derivatives of 2,4-pyrimidinediones.
Nucleosides Nucleotides Nucleic Acids 19 , 1093-1100, (2000) N-(1-alkenyl) derivatives of 2,4-pyrimidinediones (6-9) were prepared in a one pot synthesis from aldehydes and the nucleobases using trimethylsilyl trifluoromethanesulfonate (TfOTMS) as coupling reagent. Presilylation of the above nucleobases, and N6-benzoyl... |
|
|
Understanding the relationship between photolysis efficiency and metal binding using ArgenCast photocages.
Photochem. Photobiol. 88(4) , 844-50, (2012) ArgenCast-1 (1), a photocage for silver utilizing acyclic polythioether 3,6,12,15-tetrathia-9-azaheptadecane receptor and 4,5-dimethoxy-2-nitrobenzyl (DMNB) chromophore has been prepared using trimethylsilyl trifluoromethanesulfonate-assisted electrophilic ar... |
|
|
Estrone derived steroidal diepoxide: Biologically active compound and precursor of a stable steroidal A,B-spiro system
Steroids 74(12) , 890-5, (2009) A simple approach to a stable steroidal estrone derived A,B-spiro system is reported. Treatment of estrone derived A-ring diepoxyalcohol with the Ac 2O–TMSOTf system at the ambient temperature led to acetylation, while at the reflux temperature the acid-catal... |
|
|
On reactions of steroidal 23-oxo and 23,24-epoxysapogenins with Lewis acids.
Steroids 74(8) , 675-83, (2009) The reaction of 23-oxotigogenin acetate with TMSOTf in THF afforded the corresponding bisnorcholanic lactone in 60% yield. The analogous reactions carried out in dichloromethane or benzene gave the rearranged products--the isomeric spirostanic ketone (10-15%)... |
|
|
On-resin synthesis of novel arginine-isostere peptides bearing substituted amidine headgroups.
J. Pept. Sci. 18(1) , 30-6, (2012) A methodology is presented for the facile synthesis of Arg-containing peptides modified at the guanidine headgroup as substituted amidine cores. This process allows for the iterative construction of these Arg isosteres while the peptide is being built out on ... |