154-42-7
| Name | tioguanine |
|---|---|
| Synonyms |
MFCD00233553
2-Amino-6-methoxy purine tabloid 2-aminomercaptopurine 2-amino-9H-purine-6(1H)-thione tioguanin 2-Amino-6-purinethiol bw5071 Tioguanine 2-Amino-6-mercaptopurine,2-Amino-6-purinethiol 2-Amino-6-mercaptopurine 2-Amino-1H-purine-6(7H)-thione 6-Thioguanine lanvis Tg 6-tg thioguanine thio-guanin 6-thioguanidine EINECS 205-827-2 wellcomeu3b 2-Amino-1,7-dihydro-6H-purine-6-thione 6-Mercaptoguanine |
| Description | 6-Thioguanine is an anti-leukemia and immunosuppressant agent, acts as an inhibitor of SARS and MERS coronavirus papain-like proteases (PLpros) and also potently inhibits USP2 activity, with IC50s of 25 μM and 40 μM for Plpros and recombinant human USP2, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 25 μM (PLpros), 40 μM (Recombinant human USP2)[3] |
| In Vitro | 6-Thioguanine is an anti-leukemia and immunosuppressant agent, acts as an inhibitor of SARS and MERS coronavirus papain-like proteases (PLpros) and also potently inhibits USP2 activity, with IC50s of 25 μM and 40 μM for Plpros and recombinant human USP2, respectively[1]. 6-Thioguanine affects the methylation of cytosine residues by purified DNA methyltransferases including human DNMT1 and bacterial HpaII methylase. 6-Thioguanine (1 or 3 μM) decreases global cytosine methylation in Jurkat T cells and cytosine methylation in human cells at 3 μM[2]. 6-Thioguanine (18.75, 37.50, or 75.00 μM) adversely affects cell viability, but with no effect on LDH or ALT activity[3]. |
| Cell Assay | Treatments consists of 3 thiopurines (azathioprine, 6-mercaptopurine, and 6-thioguanine) at each of 6 concentrations (0.468, 0.937, 1.875, 3.750, 7.500, and 15.000 μM). Each thiopurinee is dissolved in DMSO solution to achieve a concentration of 10 mg/mL. Sterile filtered maintenance medium is used to further dilute each thiopurine solution to each of the 6 treatment concentrations. Twenty-four hours after the hepatocytes are plated on 96-well culture[3]. |
| References |
| Density | 2.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 460.7±37.0 °C at 760 mmHg |
| Melting Point | ≥300 °C(lit.) |
| Molecular Formula | C5H5N5S |
| Molecular Weight | 167.192 |
| Flash Point | 232.4±26.5 °C |
| Exact Mass | 167.026566 |
| PSA | 119.28000 |
| LogP | -0.99 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 2.071 |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S28-S36/37/39-S45-S28A |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | UP0740000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2933990090 |
|
~78% 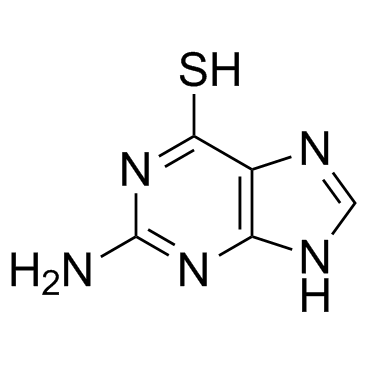
154-42-7 |
| Literature: Tomsons, U. A.; Avots, A. A.; Kolesnikova, I. A. Pharmaceutical Chemistry Journal, 1985 , vol. 19, # 2 p. 136 - 138 Khimiko-Farmatsevticheskii Zhurnal, 1985 , vol. 19, # 2 p. 206 - 209 |
|
~% 
154-42-7 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 19, # 2 p. 136 - 138 Khimiko-Farmatsevticheskii Zhurnal, , vol. 19, # 2 p. 206 - 209 |
| Precursor 2 | |
|---|---|
| DownStream 4 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |



![2-[(2-amino-5H-purin-6-yl)sulfanyl]acetamide structure](https://image.chemsrc.com/caspic/397/89853-37-2.png)
![Acetic acid,2-[(2-amino-9-propyl-9H-purin-6-yl)thio] structure](https://image.chemsrc.com/caspic/135/42204-31-9.png)