186392-40-5
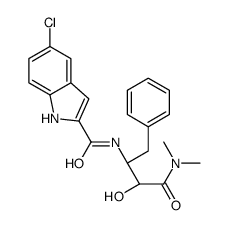
| Name | 5-chloro-N-[(2S,3R)-4-(dimethylamino)-3-hydroxy-4-oxo-1-phenylbutan-2-yl]-1H-indole-2-carboxamide |
|---|---|
| Synonyms |
UNII-O8EV00W45A
HMS3261K03 cc-263 |
| Description | CP-91149 is a GP (glycogen phosphorylase) inhibitor. CP-91149 promotes glycogen resynthesis, but not its overaccumulation. CP-91149 has the potential for Type II (insulin-dependent) diabetes study[1]. |
|---|---|
| Related Catalog | |
| In Vitro | CP-91149 treatment decreases muscle GP activity by converting the phosphorylated AMP-independent α form into the dephosphorylated AMP-dependent b form and inhibiting GP α activity and AMP-mediated GP b activation[1]. CP-91149 (10, 30, 50 μM) inhibits brain GP and causes glycogen accumulation in A549 cells[2]. Cell Viability Assay[1] Cell Line: Cells were transduced with adenoviruses and incubated in the presence of 25 mM glucose for 2 days. Concentration: 10 µM (glucose− or glucose+ for 18 h). Incubation Time: 3 h. Result: Promoted the conversion of GP a into GP b, according to α model proposed in hepatocytes. Western Blot Analysis[2] Cell Line: A549 cells. Concentration: 0, 10, 30, 50 μM. Incubation Time: 72 h. Result: A significant increase in glycogen accumulation was detected at 10 μM of CP-91149 as compared with untreated cells with a maximal glycogen accumulation at 30 μM. Intracellular glycogen content decreased at 50 μM CP-91149, perhaps explained by additional pharmacological effects of the drug. The dose-dependent accumulation of intracellular glycogen in A549 cells by CP-91149 indicates that CP-91149 inhibits brain GP in tissue culture. |
| Melting Point | 190-192°C |
|---|---|
| Molecular Formula | C21H22ClN3O3 |
| Molecular Weight | 399.87100 |
| Exact Mass | 399.13500 |
| PSA | 88.92000 |
| LogP | 3.18630 |
| Appearance | white to off-white |
| Storage condition | Refrigerator |
| Water Solubility | DMSO: >20mg/mL |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |