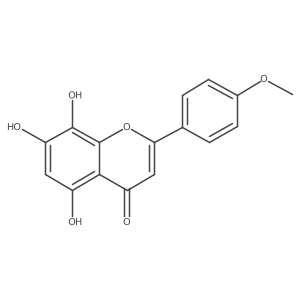
57096-02-3
| Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-8-methoxychromen-4-one |
|---|---|
| Synonyms |
4'-Hydroxywogonin
isoscutellarein-8-methylether 8-Methoxy-iso-scutellargin 5,7,4'-trihydroxy-8-methoxyflavone 4'-Hydroxywogonine 5,7-Dihydroxy-2-(4-hydroxyphenyl)-8-methoxy-4H-1-benzopyran-4-one 5,7-Dihydroxy-2-(4-hydroxyphenyl)-8-methoxy-4H-chromen-4-one isooscutellarein 8-methyl ether Isoscutellarein 8-methyl ether 8-methoxy-hebacetin |
| Description | 4′-Hydroxywogonin (8-Methoxyapigenin), a flavonoid, could be isolated from a variety of plants including Scutellaria barbata and Verbena littoralis. 4′-Hydroxywogonin has anti-inflammatory activity via TAK1/IKK/NF-κB, MAPKs and PI3/AKT signaling pathways. 4′-Hydroxywogonin inhibits angiogenesis by disrupting PI3K/AKT signaling. 4′-Hydroxywogonin inhibits cell proliferation and induces apoptosis[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | 4′-Hydroxywogonin (8-Methoxyapigenin; 0.5-15 μM; 0-24 h) has low cytotoxicity and inhibits NO and PGE2 production in LPS-stimulated RAW 264.7 macrophages by suppression of iNOS and COX-2 expression[1]. 4′-Hydroxywogonin (0.5-15 μM; 1 and 12 h) suppresses LPS-induced expression of pro-inflammatory cytokines in RAW 264.7 macrophages and suppresses LPS-induced activation of NF-κB[1]. 4′-Hydroxywogonin (0.5-15 μM; 1 h) suppresses LPS-induced degradation of IκB-α and activation of IKK and TAK and suppresses the phosphorylation of MAPK and AKTin in RAW 264.7 macrophages[1]. 4′-Hydroxywogonin (0.5-15 μM; 24 h) inhibits ROS production in LPS-stimulated RAW 264.7 macrophages[1]. 4′-Hydroxywogonin (0-10 μg/mL; 24 h) reduces the viability of SW620 cells in a concentration- and time-dependent manner and decreases the mRNA and protein expression of vascular endothelial growth factor-A (VEGF-A), the predominant pro-angiogenic cytokine in tumor angiogenesis[2]. 4′-Hydroxywogonin (24 h; SUP-B15 and Jurkat cells) induces apoptosis and decreases the expression of C-MYC, BCL-2 and cleaved caspase 3[3]. Cell Viability Assay[1] Cell Line: RAW 264.7 macrophages Concentration: 0.5, 5 and 15 μM Incubation Time: 24 hours Result: Had low cytotoxicity in RAW 264.7 macrophages. Western Blot Analysis[1] Cell Line: RAW 264.7 macrophages Concentration: 0.5, 5 and 15 μM Incubation Time: 1 hours Result: Attenuated the increase of iNOS and COX-2 mRNA expression induced by LPS in RAW 264.7 cells. Western Blot Analysis[1] Cell Line: RAW 264.7 macrophages Concentration: 0.5, 5 and 15 μM Incubation Time: 1 and 12 hours Result: Reduced TNF-α, IL-6 and IL-1β mRNA expression in a dose-dependent manner. Inhibited LPS-induced p65 phosphorylation and nuclear translocation. Western Blot Analysis[1] Cell Line: RAW 264.7 macrophages Concentration: 0.5, 5 and 15 μM Incubation Time: 1 hours Result: Attenuated LPS induced IκB-α degradation. Attenuated the phosphorylation of ERK1/2 and p38 in a dose-dependent manner. Reduced the intensity of the TAK1/TAB1 band. Western Blot Analysis[2] Cell Line: SW620 cells Concentration: 0.1, 1, and 10 μg/mL Incubation Time: 24 hours Result: Downregulated VEGF-Aexpression in colorectal cancer cells and suppressed angiogenesis. |
| In Vivo | 4′-Hydroxywogonin (10 and 20 mg/kg; i.p.; male C57BL/6 mice) alleviates LPS-induced acute lung injury (ALI) in a mouse model[1]. Animal Model: Male C57BL/6 mice (6-8 weeks old; 20 g) with acute lung injury model[1] Dosage: 10 and 20 mg/kg Administration: Intraperitoneal injection, 12 and 1 h before LPS treatment Result: Had potential protective effects against inflammation in LPS induced ALI mice. Attenuated the degree of leukocyte infiltration. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 578.1±50.0 °C at 760 mmHg |
| Molecular Formula | C16H12O6 |
| Molecular Weight | 300.263 |
| Flash Point | 220.9±23.6 °C |
| Exact Mass | 300.063385 |
| PSA | 100.13000 |
| LogP | 1.37 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.697 |
| Hazard Codes | Xi |
|---|
|
~% 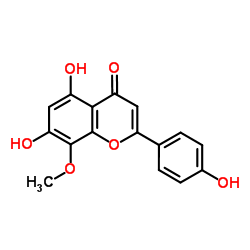
57096-02-3 |
| Literature: Brunet, Gunter; Ibrahim, Ragai K. Phytochemistry (Elsevier), 1980 , vol. 19, p. 741 - 746 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |