2',3'-Dideoxyadenosine
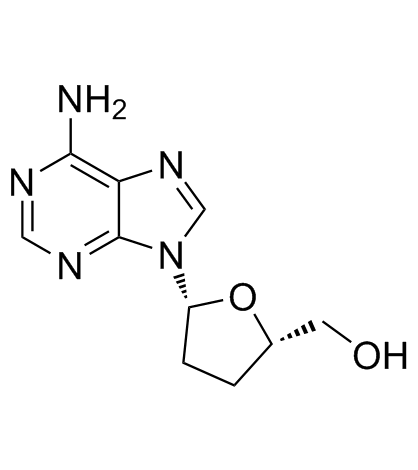
2',3'-Dideoxyadenosine structure
|
Common Name | 2',3'-Dideoxyadenosine | ||
|---|---|---|---|---|
| CAS Number | 4097-22-7 | Molecular Weight | 235.242 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 539.1±60.0 °C at 760 mmHg | |
| Molecular Formula | C10H13N5O2 | Melting Point | 181-184 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 279.9±32.9 °C | |
Use of 2',3'-Dideoxyadenosine2',3'-Dideoxyadenosine is an inhibitor of HIV replication[1]. Antiretroviral activity[1]. Antiviral efficacy[1]. |
| Name | 2',3'-Dideoxyadenosine |
|---|---|
| Synonym | More Synonyms |
| Description | 2',3'-Dideoxyadenosine is an inhibitor of HIV replication[1]. Antiretroviral activity[1]. Antiviral efficacy[1]. |
|---|---|
| Related Catalog | |
| Target |
HIV[1] |
| In Vitro | 2',3'-Dideoxyadenosine inhibits HIV-1 and simian immunodeficiency virus (SIV) in MT-4 cells with EC50s of 5.27 and 5.3 μM, respectively. 2',3'-Dideoxyadenosine inhibits HIV-1 and HIV-2 in human T-lymphocyte CEM cells with EC50s of 4 μM and 8 μM, respectively. 2',3'-Dideoxyadenosine inhibits HIV-1 and HIV-2 in Molt 4/C8 cells with EC50s of 4 μM and 5.5 μM, respectively. 2',3'-Dideoxyadenosine inhibits HIV-1 and HIV-2 in C8166 cells with EC50s of 17 μM and 22 μM, respectively. 2',3'-Dideoxyadenosine inhibits murine sarcoma virus (MSV) in C3H/3T3 cells with an EC50 of 24 μM[1]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 539.1±60.0 °C at 760 mmHg |
| Melting Point | 181-184 °C(lit.) |
| Molecular Formula | C10H13N5O2 |
| Molecular Weight | 235.242 |
| Flash Point | 279.9±32.9 °C |
| Exact Mass | 235.106918 |
| PSA | 99.08000 |
| LogP | -0.43 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.822 |
| InChIKey | WVXRAFOPTSTNLL-NKWVEPMBSA-N |
| SMILES | Nc1ncnc2c1ncn2C1CCC(CO)O1 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | C |
| Risk Phrases | R34 |
| Safety Phrases | 26-27-36/37/39-45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | AU7358900 |
| HS Code | 2934999090 |
| Precursor 7 | |
|---|---|
| DownStream 4 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
RGS19 converts iron deprivation stress into a growth-inhibitory signal
Biochem. Biophys. Res. Commun. 464 , 168-75, (2015) Iron chelation is a promising therapeutic strategy for cancer that works, in part, by inducing overexpression of N-myc downstream-regulated gene 1 protein (NDRG1), a known growth inhibitor and metasta... |
|
|
The cyclic AMP pathway is a sex-specific modifier of glioma risk in type I neurofibromatosis patients.
Cancer Res. 75(1) , 16-21, (2015) Identifying modifiers of glioma risk in patients with type I neurofibromatosis (NF1) could help support personalized tumor surveillance, advance understanding of gliomagenesis, and potentially identif... |
|
|
Metformin as adjunct antituberculosis therapy.
Sci. Transl. Med. 6(263) , 263ra159, (2014) The global burden of tuberculosis (TB) morbidity and mortality remains immense. A potential new approach to TB therapy is to augment protective host immune responses. We report that the antidiabetic d... |
| DDA |
| ((2S,5R)-5-(6-Amino-9H-purin-9-yl)tetrahydrofuran-2-yl)methanol |
| [(2S,5R)-5-(6-aminopurin-9-yl)oxolan-2-yl]methanol |
| Dideoxyadenosine |
| Didanosine impurity G |
| 6-Amino-9-(2',3'-dideoxy-b-D-glycero-pentofuranosyl)purine |
| EINECS 223-853-2 |
| [(2S,5R)-5-(6-Amino-9H-purin-9-yl)tetrahydrofuran-2-yl]methanol |
| 2-furanmethanol, 5-(6-amino-9H-purin-9-yl)tetrahydro-, (2S,5R)- |
| 2',3'-Dideoxyadenosine |
| 9-(2,3-Dideoxy-β-D-ribofuranosyl)adenine |
| MFCD00010534 |
| 9-(2,3-Dideoxy-beta-D-ribofuranosyl)adenine |
| [(2S,5R)-5-(6-Amino-9H-purin-9-yl)tetrahydro-2-furanyl]methanol |
 CAS#:7057-48-9
CAS#:7057-48-9 CAS#:66224-66-6
CAS#:66224-66-6 CAS#:5983-09-5
CAS#:5983-09-5 CAS#:205189-73-7
CAS#:205189-73-7 CAS#:58-61-7
CAS#:58-61-7 CAS#:32780-06-6
CAS#:32780-06-6![5'-O-[(1,1-dimethylethyl)dimethylsilyl]adenosine Structure](https://image.chemsrc.com/caspic/287/69530-93-4.png) CAS#:69530-93-4
CAS#:69530-93-4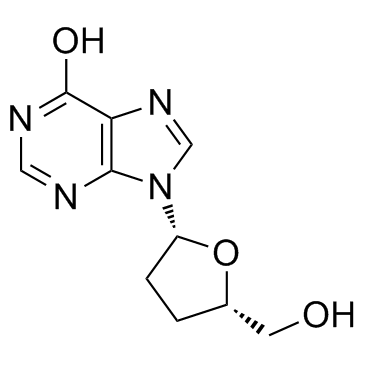 CAS#:69655-05-6
CAS#:69655-05-6![[(2S,5R)-5-purin-9-yloxolan-2-yl]methanol structure](https://image.chemsrc.com/caspic/473/126502-08-7.png) CAS#:126502-08-7
CAS#:126502-08-7![[(2S,5R)-5-(6-amino-8-bromopurin-9-yl)oxolan-2-yl]methanol structure](https://image.chemsrc.com/caspic/189/121353-86-4.png) CAS#:121353-86-4
CAS#:121353-86-4
