Podophyllotoxin
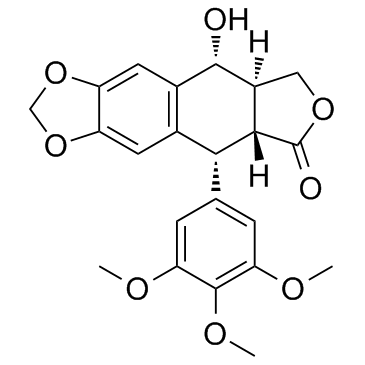
Podophyllotoxin structure
|
Common Name | Podophyllotoxin | ||
|---|---|---|---|---|
| CAS Number | 518-28-5 | Molecular Weight | 414.405 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 597.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H22O8 | Melting Point | 183-184 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 210.2±23.6 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of PodophyllotoxinPodophyllotoxin is a potent inhibitor of microtubule assembly and DNA topoisomerase II.IC50 Value:Target: Topoisomerase II; Microtubule/TubulinPodophyllotoxin, a kind of non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum plant, has been shown to inhibit the growth of various carcinoma cells. Podophyllotoxin is a natural product that inhibits the polymerization of tubulin and has served as a prototype for the development of diverse antitumor agents in clinical use. |
| Name | podophyllotoxin |
|---|---|
| Synonym | More Synonyms |
| Description | Podophyllotoxin is a potent inhibitor of microtubule assembly and DNA topoisomerase II.IC50 Value:Target: Topoisomerase II; Microtubule/TubulinPodophyllotoxin, a kind of non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum plant, has been shown to inhibit the growth of various carcinoma cells. Podophyllotoxin is a natural product that inhibits the polymerization of tubulin and has served as a prototype for the development of diverse antitumor agents in clinical use. |
|---|---|
| Related Catalog | |
| References |
[5]. Podophyllotoxin |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 597.9±50.0 °C at 760 mmHg |
| Melting Point | 183-184 °C(lit.) |
| Molecular Formula | C22H22O8 |
| Molecular Weight | 414.405 |
| Flash Point | 210.2±23.6 °C |
| Exact Mass | 414.131470 |
| PSA | 92.68000 |
| LogP | 1.60 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.606 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H310-H315-H319-H335 |
| Precautionary Statements | P261-P280-P302 + P350-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R23/25 |
| Safety Phrases | S36/37/39-S45 |
| RIDADR | UN 3462 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | LV2500000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2942000000 |
| Precursor 4 | |
|---|---|
| DownStream 9 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
| (5R,5aR,8aR,9R)-5,8,8a,9-Tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one |
| EINECS 208-250-4 |
| Podofilox |
| Podophyllotoxin |
| (5R,5aR,8aR,9R)-9-Hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5aH)-one |
| Wartec |
| 1-Hydroxy-2-hydroxymethyl-6,7-methylenedioxy-4-(3',4',5'-trimethoxyphenyl)-1,2,3,4-tetrahydronaphthalene-3-carboxylic Acid Lactone |
| Condylox |
| Condyline |
| Podophyllotoxin (8CI) |
| (5R,5aR,8aR,9R)-5-hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one |
| Warticon |
| [5R-(5a,5ab,8aa,9a)]-5,8,8a,9-tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one |
| MFCD00075290 |
| Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 5,8,8a,9-tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-, (5R,5aR,8aR,9R)- |
| Podophyllinic acid lactone |
 CAS#:112711-16-7
CAS#:112711-16-7 CAS#:74879-22-4
CAS#:74879-22-4 CAS#:87850-43-9
CAS#:87850-43-9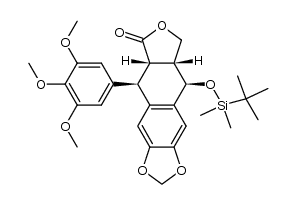 CAS#:108448-52-8
CAS#:108448-52-8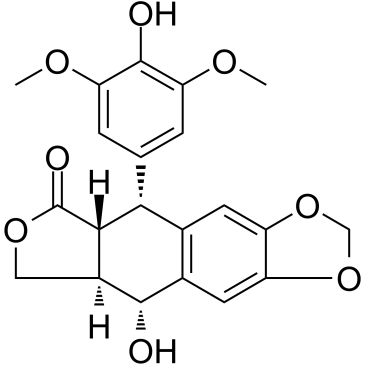 CAS#:40505-27-9
CAS#:40505-27-9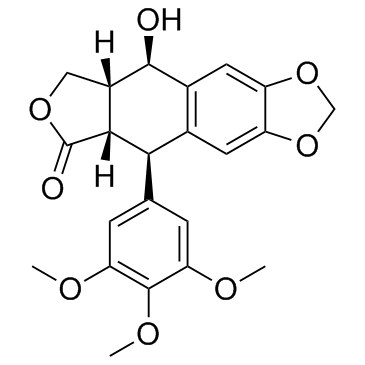 CAS#:477-47-4
CAS#:477-47-4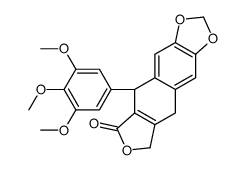 CAS#:477-52-1
CAS#:477-52-1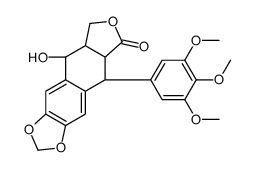 CAS#:4375-07-9
CAS#:4375-07-9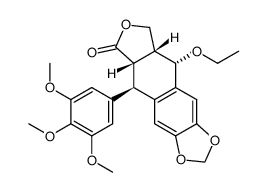 CAS#:78215-99-3
CAS#:78215-99-3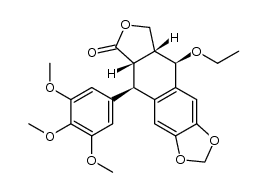 CAS#:245321-94-2
CAS#:245321-94-2 CAS#:6559-91-7
CAS#:6559-91-7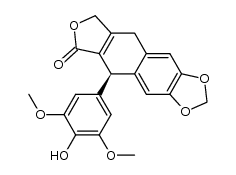 CAS#:102306-96-7
CAS#:102306-96-7 CAS#:102306-95-6
CAS#:102306-95-6
