2,6-Dihydroxypurine
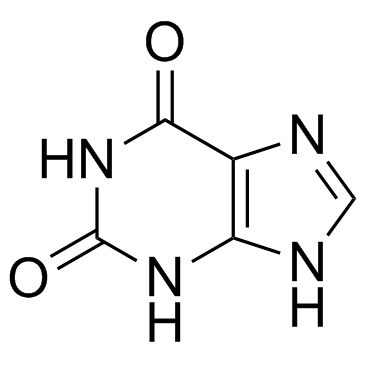
2,6-Dihydroxypurine structure
|
Common Name | 2,6-Dihydroxypurine | ||
|---|---|---|---|---|
| CAS Number | 69-89-6 | Molecular Weight | 152.111 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 834.9ºC at 760 mmHg | |
| Molecular Formula | C5H4N4O2 | Melting Point | 300 °C | |
| MSDS | Chinese USA | Flash Point | 458.7ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2,6-DihydroxypurineXanthine is a purine base found in most human body tissues and fluids and in other organisms. |
| Name | 7H-xanthine |
|---|---|
| Synonym | More Synonyms |
| Description | Xanthine is a purine base found in most human body tissues and fluids and in other organisms. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | A number of stimulants are derived from Xanthine including caffeine and theobromine. Xanthine is a product on the pathway of purine degradation. Xanthine is subsequently converted to uric acid by the action of the Xanthine oxidase enzyme. |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 834.9ºC at 760 mmHg |
| Melting Point | 300 °C |
| Molecular Formula | C5H4N4O2 |
| Molecular Weight | 152.111 |
| Flash Point | 458.7ºC |
| Exact Mass | 152.033432 |
| PSA | 94.40000 |
| LogP | -0.81 |
| Index of Refraction | 1.636 |
| InChIKey | LRFVTYWOQMYALW-UHFFFAOYSA-N |
| SMILES | O=c1[nH]c(=O)c2[nH]cnc2[nH]1 |
| Storage condition | Store at RT. |
| Water Solubility | NH4OH: freely soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H319 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36;R43 |
| Safety Phrases | S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | ZD7700000 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Fungal metabolite nigerloxin ameliorates diabetic nephropathy and gentamicin-induced renal oxidative stress in experimental rats.
Naunyn Schmiedebergs Arch. Pharmacol. 387(9) , 849-59, (2014) Elevated polyol pathway enzyme activities and oxidative stress play an important role in the development and progression of diabetic nephropathy. Here, we investigated the beneficial influence of nige... |
|
|
Antioxidant Capacities and Analysis of Phenolic Compounds in Three Endemic Nolana Species by HPLC-PDA-ESI-MS.
Molecules 20 , 11490-507, (2015) The antioxidant features, polyphenolic composition and chromatographic fingerprints of the aerial parts from three Chilean endemic plants from the Paposo Valley located on the cost of the Atacama Dese... |
|
|
Spironolactone and dimethylsulfoxide effect on glucose metabolism and oxidative stress markers in polycystic ovarian syndrome rat model.
Exp. Clin. Endocrinol. Diabetes 122(3) , 154-62, (2014) Because polycystic ovarian syndrome (PCOS) is a risk factor for type 2 diabetes, the affected women can present frequently prediabetic states such as impaired fasting glycaemia and/or impaired glucose... |
| UREOUS ACID |
| Xan |
| xanthicoxide |
| Dioxopurine |
| 9H-xanthine |
| 2,5-DIFLUOROTHIOBENZAMIDE |
| usafcb-17 |
| Xanthine |
| 9H-Purine-2,6(1H,3H)-dione |
| Purine-2,6(1H,3H)-dione |
| Xanthin |
| ISOXANTHINE |
| 2,6-Dihydroxypurine |
| MFCD00078453 |
| 3,7-dihydropurine-2,6-dione |
| Xanthione |
| EINECS 200-718-6 |
| 3,7-dihydro-1H-purine-2,6-dione |
| 1H-Purine-2,6-dione, 3,7-dihydro- |
| 3,7-dihydro-purine-2,6-dione |
| 2,6(1H,3H)-Purinedione |
 CAS#:73-40-5
CAS#:73-40-5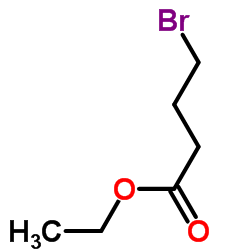 CAS#:2969-81-5
CAS#:2969-81-5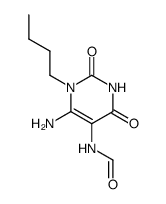 CAS#:76194-10-0
CAS#:76194-10-0 CAS#:69-93-2
CAS#:69-93-2 CAS#:6979-73-3
CAS#:6979-73-3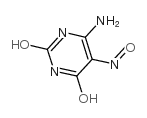 CAS#:5442-24-0
CAS#:5442-24-0 CAS#:77287-34-4
CAS#:77287-34-4 CAS#:290-87-9
CAS#:290-87-9 CAS#:3240-72-0
CAS#:3240-72-0 CAS#:146-80-5
CAS#:146-80-5 CAS#:20419-68-5
CAS#:20419-68-5 CAS#:83-67-0
CAS#:83-67-0 CAS#:58-08-2
CAS#:58-08-2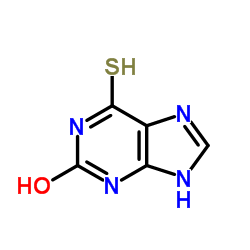 CAS#:2002-59-7
CAS#:2002-59-7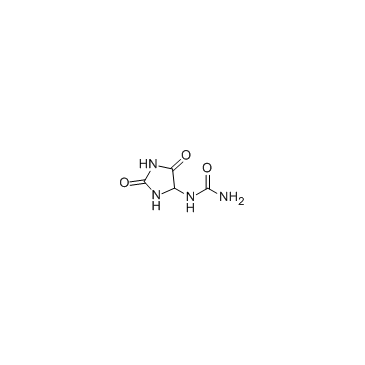 CAS#:97-59-6
CAS#:97-59-6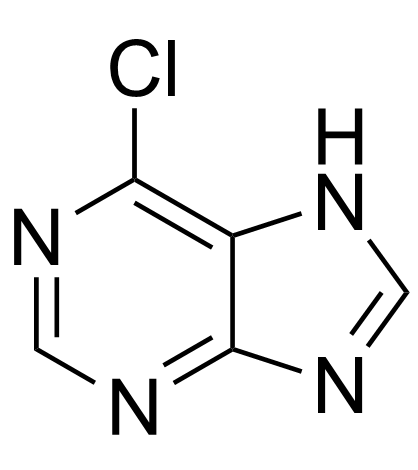 CAS#:87-42-3
CAS#:87-42-3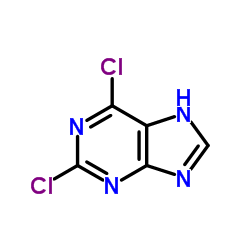 CAS#:5451-40-1
CAS#:5451-40-1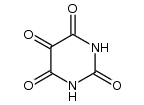 CAS#:61066-33-9
CAS#:61066-33-9
