Etidronate disodium
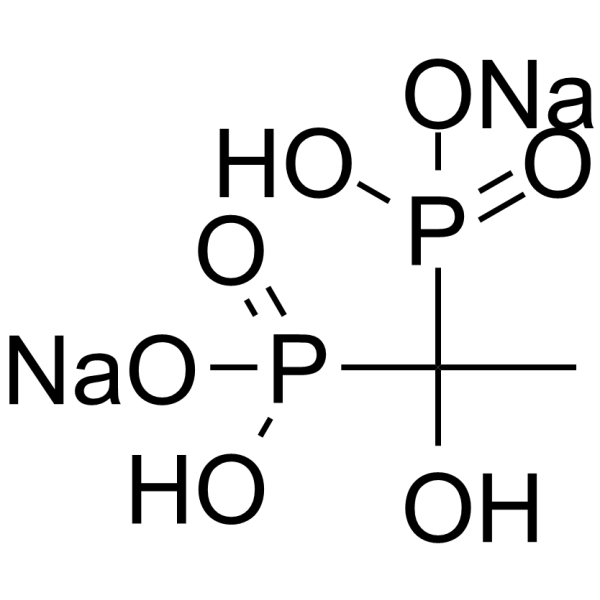
Etidronate disodium structure
|
Common Name | Etidronate disodium | ||
|---|---|---|---|---|
| CAS Number | 7414-83-7 | Molecular Weight | 249.992 | |
| Density | N/A | Boiling Point | 578.8ºC at 760 mmHg | |
| Molecular Formula | C2H6Na2O7P2 | Melting Point | > 300ºC | |
| MSDS | Chinese USA | Flash Point | 303.8ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Etidronate disodiumEtidronic acid (Etidronate) disodium is an orally and intravenously active bisphosphonate. Etidronic acid disodium inhibits resorption of bone, reduces arterial calcification and can be used for osteoporosis research. Etidronic acid disodium has anticancer activity. Etidronic acid disodium is a chelating agent and can be used to remove heavy metal in water[1][2][3][4]. |
| Name | etidronate disodium |
|---|---|
| Synonym | More Synonyms |
| Description | Etidronic acid (Etidronate) disodium is an orally and intravenously active bisphosphonate. Etidronic acid disodium inhibits resorption of bone, reduces arterial calcification and can be used for osteoporosis research. Etidronic acid disodium has anticancer activity. Etidronic acid disodium is a chelating agent and can be used to remove heavy metal in water[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Etidronic acid (Etidronate) (10 mM, 24 h) disodium shows cytotoxicity, causes a decrease in the S-phase population and an increase in the G2/M population, induces p53 mutation in MCF-7 cells[3]. Etidronic acid (100 nM, 24 h) disodium induces osteoclast apoptosis, which displays characteristics of apoptosis, including chromatin condensation and changed cellular and nuclear morphology, detected by fluorescence microscopy[5]. |
| References |
| Boiling Point | 578.8ºC at 760 mmHg |
|---|---|
| Melting Point | > 300ºC |
| Molecular Formula | C2H6Na2O7P2 |
| Molecular Weight | 249.992 |
| Flash Point | 303.8ºC |
| Exact Mass | 249.938416 |
| PSA | 160.57000 |
| InChIKey | GWBBVOVXJZATQQ-UHFFFAOYSA-L |
| SMILES | CC(O)(P(=O)([O-])O)P(=O)([O-])O.[Na+].[Na+] |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 26 mg/mL | Soluble in water (26 mg/ml). |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
Effects of various squalene epoxides on coenzyme Q and cholesterol synthesis.
Biochim. Biophys. Acta 1841(7) , 977-86, (2014) 2,3-Oxidosqualene is an intermediate in cholesterol biosynthesis and 2,3:22,23-dioxidosqualene act as the substrate for an alternative pathway that produces 24(S),25-epoxycholesterol which effects cho... |
|
|
Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial.
Lancet Oncol. 15(13) , 1460-8, (2014) Aromatase inhibitors prevent breast cancer in postmenopausal women at high risk of the disease but are associated with accelerated bone loss. We assessed effectiveness of oral risedronate for preventi... |
|
|
A novel automated hydrophilic interaction liquid chromatography method using diode-array detector/electrospray ionization tandem mass spectrometry for analysis of sodium risedronate and related degradation products in pharmaceuticals
J. Chromatogr. A. 1365 , 131-9, (2014) • HPLC fused core technology to analyze SR and its degradation products. • Stress test studies on raw material, finished products and placebos. • No interference of the complex matrices (new pharmaceu... |
| Disodium (1-hydroxy-1,1-ethanediyl)bis[hydrogen (phosphonate)] |
| EINECS 231-025-7 |
| Disodium Etidronate Hydrate |
| MFCD00152567 |
| Didronel |
| Dinatrium-(1-hydroxyethan-1,1-diyl)bis[hydrogen-(phosphonat)] |
| 1-Hydroxyethylidene-1,1-diphosphonic acid disodium salt |
| Etidronate disodium |
| Disodium (1-hydroxyethane-1,1-diyl)bis[hydrogen (phosphonate)] |
| Phosphonic acid, (1-hydroxyethylidene)bis-, sodium salt (1:2) |
| disodium etidronate |
| Disodium (1-hydroxyethylidene)diphosphonate |
| disodium,hydroxy-[1-hydroxy-1-[hydroxy(oxido)phosphoryl]ethyl]phosphinate |
| Disodium 1-hydroxyethylidene phosphonate |
| (1-Hydroxyethylidene)diphosphonic acid, disodium salt |
| (1-hydroxyéthane-1,1-diyl)bis[hydrogène (phosphonate)] de disodium |

