BML-278
Modify Date: 2024-01-02 11:57:17
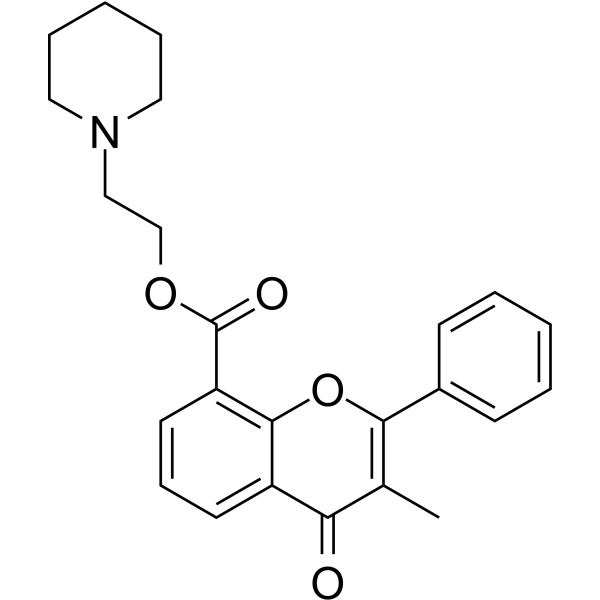
BML-278 structure
|
Common Name | BML-278 | ||
|---|---|---|---|---|
| CAS Number | 15301-69-6 | Molecular Weight | 391.460 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 564.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H25NO4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 294.9±30.1 °C | |
Use of BML-278Flavoxate is a potent and competitive phosphodiesterase (PDE) inhibitor. Flavoxate is an antispasmodic agent and muscarinic mAChR antagonist. Flavoxate shows moderate calcium antagonistic activity and local anesthetic effect. Flavoxate can be used for the research of overactive bladder (OAB) and lower urinary tract infections[1][2]. |
| Name | flavoxate |
|---|---|
| Synonym | More Synonyms |
| Description | Flavoxate is a potent and competitive phosphodiesterase (PDE) inhibitor. Flavoxate is an antispasmodic agent and muscarinic mAChR antagonist. Flavoxate shows moderate calcium antagonistic activity and local anesthetic effect. Flavoxate can be used for the research of overactive bladder (OAB) and lower urinary tract infections[1][2]. |
|---|---|
| Related Catalog | |
| References |
[1]. Arcaniolo D, et al. Flavoxate: present and future. Eur Rev Med Pharmacol Sci. 2015;19(5):719-31. |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 564.1±50.0 °C at 760 mmHg |
| Molecular Formula | C24H25NO4 |
| Molecular Weight | 391.460 |
| Flash Point | 294.9±30.1 °C |
| Exact Mass | 391.178345 |
| PSA | 59.75000 |
| LogP | 5.18 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.591 |
|
~96% 
BML-278 CAS#:15301-69-6 |
| Literature: Yamamoto Chemical Industrial Co., Ltd.; Osaka Municipal Government Patent: US4634768 A1, 1987 ; |
|
~92% 
BML-278 CAS#:15301-69-6 |
| Literature: Yamamoto Chemical Industrial Company, Limited; Osaka Municipal Government Patent: US4533732 A1, 1985 ; |
|
~80% 
BML-278 CAS#:15301-69-6 |
| Literature: Yamamoto Chemical Industrial Co., Ltd.; Osaka Municipal Government Patent: US4634768 A1, 1987 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| flavoxate |
| 3-Methylflavone-8-carboxylic Acid b-Piperidinoethyl Ester |
| Genurin |
| Flavossato [DCIT] |
| Flavoxatum [INN-Latin] |
| 3-Methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylic Acid 2-(1-Piperidinyl)ethyl Ester |
| Flavoxato [INN-Spanish] |
| MFCD00210291 |
| Flavossato |
| Flavoxate [INN:BAN] |
| Flavoxatum |
| REC 7-0040 |
| 2-(Piperidin-1-yl)ethyl-3-methyl-4-oxo-2-phenyl-4H-chromen-8-carboxylat |
| 2-(Piperidin-1-yl)ethyl 3-methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylate |
| 2-piperidin-1-ylethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate |
| 2-(1-Piperidinyl)ethyl 3-methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylate |
| Urispas |
| 2-piperidin-1-ylethyl 3-methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylate |
| 2-Piperidinoethyl 3-Methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate |
| EINECS 239-337-5 |
| Bladderon |
| Spasuret |
| 2-Piperidinoethyl 3-Methylflavone-8-carboxylate |
| Flavoxato |
| 4H-1-Benzopyran-8-carboxylic acid, 3-methyl-4-oxo-2-phenyl-, 2-(1-piperidinyl)ethyl ester |