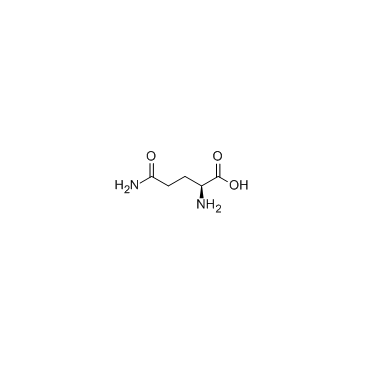
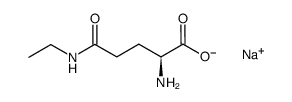
56-85-9
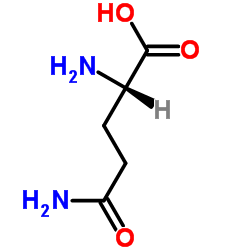
| Name | L-glutamine |
|---|---|
| Synonyms |
UNII-U5JDO2770Z
EINECS 200-292-1 (2S)-2-((2S)-2-Aminopropanoylamino)-4-carbamoylbutanoic acid ZY1&VMYVQ2VZ &&L-L Form glutaminic acid Gln L-Ala-L-Gln glutamine L-Gln L-Alanyl-L-glutamine Levoglutamide (S)-5-Amino-2-[(S)-2-aminopropanamido]-5-oxopentanoic acid L-Glutamine,L-alanyl (2S)-2-amino-4-carbamoylbutanoic acid Alanyl-glutamine,Glutamine-S MFCD00008044 Ala-Gln L-(+)-Glutamine (S)-(+)-Glutamine 5-Hydroxy-5-imino-L-norvaline N(2)-L-alanyl-L-glutamine Glutamine-S Alanyl-glutamine L-Glutamic Acid g-Amide L-Glutamic acid γ-amide N-L-alanyl-L-glutamine L-Glutamine l-alanyl-l-glutamin l-(+)-glutamic acid-5-amide L-Glutamic acid 5-amide H-Ala-Gln-OH (2S)-5-Amino-2-{[(2S)-2-aminopropanoyl]amino}-5-oxopentanoic acid S(+)-Glutamic acid 5-amide H-Gln-OH |
| Description | L-Glutamine is a non-essential amino acid present abundantly throughout the body and is involved in gastrointestinal disorders.Target: mGluRGlutamine (abbreviated as Gln or Q) is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid, but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders. Its side-chain is an amide formed by replacing the side-chain hydroxyl of glutamic acid with an amine functional group, making it the amide of glutamic acid. Its codons are CAA and CAG. In human blood, glutamine is the most abundant free amino acid, with a concentration of about 500-900 μmol/L. Glutamine is synthesized by the enzyme glutamine synthetase from glutamate and ammonia. The most relevant glutamine-producing tissue is the muscle mass, accounting for about 90% of all glutamine synthesized. Glutamine is also released, in small amounts, by the lung and the brain. Although the liver is capable of relevant glutamine synthesis, its role in glutamine metabolism is more regulatory than producing, since the liver takes up large amounts of glutamine derived from the gut. The most eager consumers of glutamine are the cells of intestines, the kidney cells for the acid-base balance, activated immune cells, and manycancer cells. In respect to the last point mentioned, different glutamine analogues, such as DON, Azaserine or Acivicin, are tested as anticancer drugs. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 353.5±52.0 °C at 760 mmHg |
| Melting Point | 185ºC |
| Molecular Formula | C5H10N2O3 |
| Molecular Weight | 146.145 |
| Flash Point | 167.6±30.7 °C |
| Exact Mass | 146.069138 |
| PSA | 106.41000 |
| LogP | -1.28 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.564 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | MA2275100 |
| HS Code | 2932999099 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |