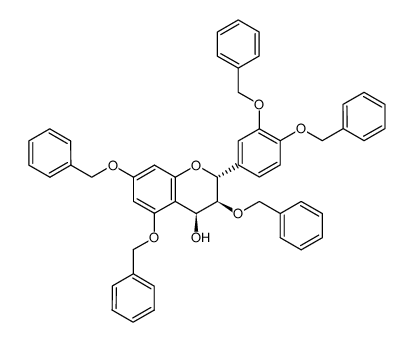
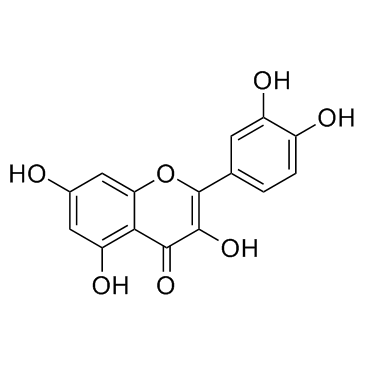
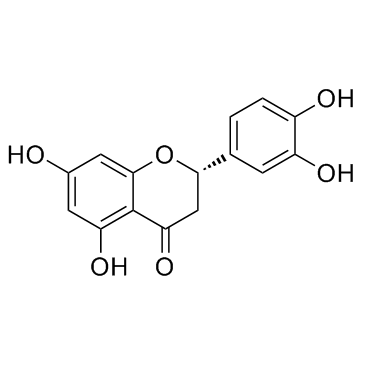
480-18-2
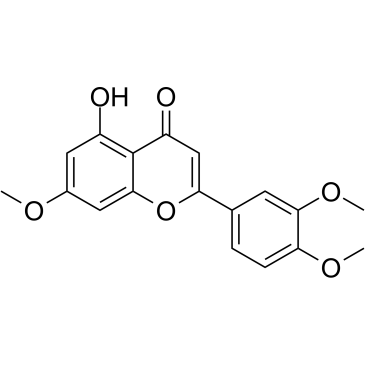
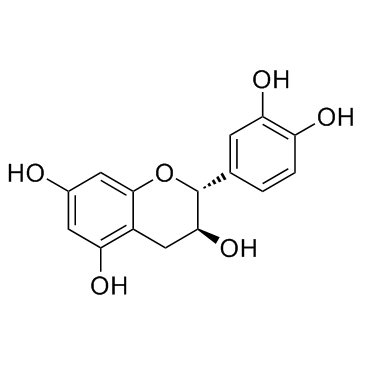
| Name | (+)-taxifolin |
|---|---|
| Synonyms |
EINECS 207-543-4
Dihydroquercetin (3R)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one (2R,3R)-3,3',4',5,7-Pentahydroxyflavanone Distylin (+)-Taxifolin (2S,3S)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one Taxifolin MFCD00006845 (-)-taxifolin Taxifoliol (2R,3R)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one |
| Description | Taxifolin exhibits important anti-tyrosinase activity. Taxifolin exhibits significant inhibitory activity against collagenase with an IC50 value of 193.3 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 193.3 μM (Collagenase)[1] Tyrosinase[1] |
| In Vitro | This is confirmed by the investigation of pure Taxifolin and (+)-Catechin against collagenase activity. Taxifolin exhibits significant inhibitory activity with an IC50 value of 193.3 μM while (+)-Catechin is not active[1]. Taxifolin is a ubiquitous bioactive constituent of foods and herbs. Taxifolin (dihydroquercetin) is a bioactive flavanonol commonly found in grapes, citrus fruits, onions, green tea, olive oil, wine, and many other foods, as well as several herbs (such as milk thistle, French maritime bark, Douglas fir bark, and Smilacis Glabrae Rhizoma)[2]. |
| In Vivo | Taxifolin may be easily metabolized and that its metabolites are the prevalent form in vivo, although limited information is available on metabolism of Taxifolin in vivo[2]. |
| Animal Admin | Rats[2] Twelve male Sprague-Dawley rats (weighing 180-220 g) are used. The rats are randomly divided into two groups (six rats per group), a drug group and a blank group. Taxifolin is suspended in 0.5% CMC-Na solution and orally administered to the drug group at a dose of 200 mg/kg body weight, while blank group rats are orally administered 0.5% CMC-Na solution at the same volume. All rats are dosed once a day (at 9:00 a.m.) for 3 days. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 687.6±55.0 °C at 760 mmHg |
| Melting Point | 230-233°C (dec.) |
| Molecular Formula | C15H12O7 |
| Molecular Weight | 304.252 |
| Flash Point | 264.2±25.0 °C |
| Exact Mass | 304.058289 |
| PSA | 127.45000 |
| LogP | 1.82 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.763 |
| Storage condition | -20?C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LK6920000 |
| HS Code | 2932999099 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




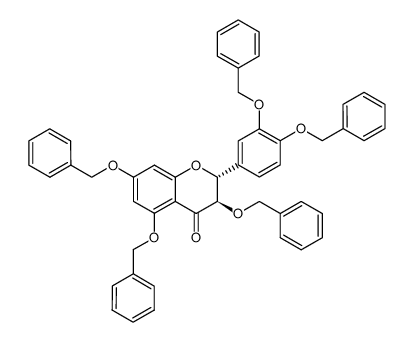

![(2R-trans)-2-[3,4-bis(phenylmethoxy)phenyl]-3,4-dihydro-3,5,7-tris(phenylmethoxy)-2H-1-benzopyran structure](https://image.chemsrc.com/caspic/447/85443-49-8.png)