S-Methyl-L-cysteine
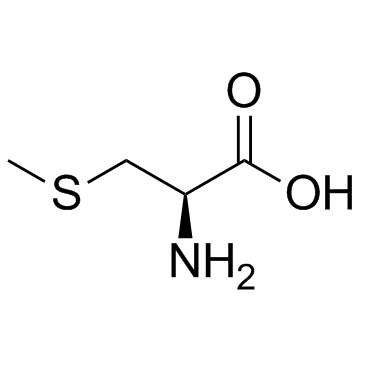
S-Methyl-L-cysteine structure
|
Common Name | S-Methyl-L-cysteine | ||
|---|---|---|---|---|
| CAS Number | 1187-84-4 | Molecular Weight | 135.185 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 242.8±30.0 °C at 760 mmHg | |
| Molecular Formula | C4H9NO2S | Melting Point | ~240 °C (dec.) | |
| MSDS | Chinese USA | Flash Point | 100.7±24.6 °C | |
Use of S-Methyl-L-cysteineS-Methyl-L-cysteine is a natural product that acts as a substrate in the catalytic antioxidant system mediated by methionine sulfoxide reductase A (MSRA), with antioxidative, neuroprotective, and anti-obesity activities. |
| Name | S-methyl-L-cysteine |
|---|---|
| Synonym | More Synonyms |
| Description | S-Methyl-L-cysteine is a natural product that acts as a substrate in the catalytic antioxidant system mediated by methionine sulfoxide reductase A (MSRA), with antioxidative, neuroprotective, and anti-obesity activities. |
|---|---|
| Related Catalog | |
| In Vivo | S-Methyl-L-cysteine (100 mg/kg) results in significant attenuation of plasma glucose, insulin, tumor necrosis factor-alpha, insulin resistance and improved antioxidant enzyme activities[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 242.8±30.0 °C at 760 mmHg |
| Melting Point | ~240 °C (dec.) |
| Molecular Formula | C4H9NO2S |
| Molecular Weight | 135.185 |
| Flash Point | 100.7±24.6 °C |
| Exact Mass | 135.035400 |
| PSA | 88.62000 |
| LogP | 0.47 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.509 |
| InChIKey | IDIDJDIHTAOVLG-VKHMYHEASA-N |
| SMILES | CSCC(N)C(=O)O |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25-S36/37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
|
Selective Allosteric Inhibition of MMP9 Is Efficacious in Preclinical Models of Ulcerative Colitis and Colorectal Cancer.
PLoS ONE 10 , e0127063, (2015) Expression of matrix metalloproteinase 9 (MMP9) is elevated in a variety of inflammatory and oncology indications, including ulcerative colitis and colorectal cancer. MMP9 is a downstream effector and... |
|
|
Cysteine amide adduct formation from carboxylic acid drugs via UGT-mediated bioactivation in human liver microsomes.
Pharmazie 70 , 678-83, (2015) Although chemical trapping has been widely used to evaluate cytochrome P450-mediated drug bioactivation, thus far, only a few in vitro-trapping studies have been performed on UDP-glucuronosyltransfera... |
|
|
Synthesis and biological evaluation of L-cysteine derivatives as mitotic kinesin Eg5 inhibitors.
Bioorg. Med. Chem. Lett. 17 , 3921-4, (2007) Inhibition of Eg5 represents a novel approach for the treatment of cancer. Here, we report the synthesis and structure-activity relationship of S-trityl-L-cysteine (STLC) derivatives as Eg5 inhibitors... |
| L-methylcysteine |
| (2R)-2-(Methylamino)-3-sulfanylpropanoic acid |
| S-METHYL-CYSTEINE |
| H-Cys(Me)-OH |
| SMC |
| Methylcysteine |
| DL-S-Methyl-cysteine |
| N-Methyl-L-cystein |
| Cysteine, N-methyl, L- |
| (R)-N-Methylcysteine |
| S-Methyl-L-cysteine |
| (2R)-2-amino-3-methylsulfanyl-propionic acid |
| EINECS 214-701-6 |
| L-Cysteine, N-methyl- |
| L-Cysteine,S-methyl |
| N-Méthyl-L-cystéine |
| L-CH3SCH2CH(NH2)COOH |
| N-Methyl-L-cysteine |
| H-L-CYS(ME)-OH |
| MFCD00002612 |
| (R)-2-Amino-3-(methylmercapto)propionic acid SMLC |
| S-Methyl-L-Cys-OH |
 CAS#:34017-27-1
CAS#:34017-27-1 CAS#:52-90-4
CAS#:52-90-4 CAS#:74-88-4
CAS#:74-88-4 CAS#:16637-59-5
CAS#:16637-59-5 CAS#:52-89-1
CAS#:52-89-1 CAS#:616-38-6
CAS#:616-38-6 CAS#:18412-81-2
CAS#:18412-81-2 CAS#:64969-77-3
CAS#:64969-77-3 CAS#:50-89-5
CAS#:50-89-5 CAS#:108-95-2
CAS#:108-95-2 CAS#:19651-44-6
CAS#:19651-44-6 CAS#:74-93-1
CAS#:74-93-1 CAS#:75-18-3
CAS#:75-18-3 CAS#:56-41-7
CAS#:56-41-7 CAS#:56-45-1
CAS#:56-45-1 CAS#:56-84-8
CAS#:56-84-8 CAS#:6853-87-8
CAS#:6853-87-8![L-Alanine, 3-[(R)-methylsulfinyl]- (9CI) structure](https://image.chemsrc.com/caspic/041/3226-62-8.png) CAS#:3226-62-8
CAS#:3226-62-8 CAS#:100-61-8
CAS#:100-61-8
