Repaglinide

Repaglinide structure
|
Common Name | Repaglinide | ||
|---|---|---|---|---|
| CAS Number | 135062-02-1 | Molecular Weight | 452.586 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 672.9±55.0 °C at 760 mmHg | |
| Molecular Formula | C27H36N2O4 | Melting Point | 129-130.2 °C | |
| MSDS | Chinese USA | Flash Point | 360.8±31.5 °C | |
Use of RepaglinideRepaglinide(AG-EE 623ZW) is a carbamoylmethyl benzoic acid (CMBA) derivative, which recently has become available for the treatment of type II diabetes. IC50 value:Target: Repaglinide is very rapidly absorbed (tmax less than 1 hour) with a t1/2 of less than one hour. Furthermore, repaglinide is inactivated in the liver and more than 90 % excreted via the bile. Repaglinide (1 mg/kg p.o.) was effective (P < 0.001) as an insulin-releasing agent in a rat model (low-dose streptozotocin) of type 2 diabetes. |
| Name | Repaglinide |
|---|---|
| Synonym | More Synonyms |
| Description | Repaglinide(AG-EE 623ZW) is a carbamoylmethyl benzoic acid (CMBA) derivative, which recently has become available for the treatment of type II diabetes. IC50 value:Target: Repaglinide is very rapidly absorbed (tmax less than 1 hour) with a t1/2 of less than one hour. Furthermore, repaglinide is inactivated in the liver and more than 90 % excreted via the bile. Repaglinide (1 mg/kg p.o.) was effective (P < 0.001) as an insulin-releasing agent in a rat model (low-dose streptozotocin) of type 2 diabetes. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 672.9±55.0 °C at 760 mmHg |
| Melting Point | 129-130.2 °C |
| Molecular Formula | C27H36N2O4 |
| Molecular Weight | 452.586 |
| Flash Point | 360.8±31.5 °C |
| Exact Mass | 452.267517 |
| PSA | 78.87000 |
| LogP | 4.69 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.568 |
| InChIKey | FAEKWTJYAYMJKF-QHCPKHFHSA-N |
| SMILES | CCOc1cc(CC(=O)NC(CC(C)C)c2ccccc2N2CCCCC2)ccc1C(=O)O |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 34 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | 000000033825 |
| HS Code | 2933399090 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
[symbol: see text] Nateglinide and [symbol: see text] repaglinide for type 2 diabetes?
Drug Ther. Bull. 41(7) , 52-4, (2003) [symbol: see text] Nateglinide (Starlix-Novartis) and [symbol: see text] repaglinide (NovoNorm-Novo Nordisk) are two of a new class of orally active antidiabetic drugs, the meglitinides. They have a r... |
|
|
Drug-drug and food-drug pharmacokinetic interactions with new insulinotropic agents repaglinide and nateglinide.
Clin. Pharmacokinet. 46(2) , 93-108, (2007) This review describes the current knowledge on drug-drug and food-drug interactions with repaglinide and nateglinide. These two meglitinide derivatives, commonly called glinides, have been developed f... |
|
|
Analysis of the repaglinide concentration increase produced by gemfibrozil and itraconazole based on the inhibition of the hepatic uptake transporter and metabolic enzymes.
Drug Metab. Dispos. 41(2) , 362-71, (2013) The plasma concentration of repaglinide is reported to increase greatly when given after repeated oral administration of itraconazole and gemfibrozil. The present study analyzed this interaction based... |
| (S)-2-Ethoxy-4-(2-((3-methyl-1-(2-(piperidin-1-yl)phenyl)butyl)amino)-2-oxoethyl)benzoic acid |
| (S)-(+)-2-Ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoic acid |
| (S)-2-ethoxy-4-(2-(3-methyl-1-(2-(piperidin-1-yl)phenyl)butylamino)-2-oxoethyl)benzoic acid |
| Repaglinid |
| 2-Ethoxy-4-[2-[[(1S)-3-Methyl-1-[2-(1-Piperidinyl)Phenyl]Butyl]Amino]-2-Oxoethyl]Benzoic Acid |
| AG EE-623 ZW |
| (+)-2-Ethoxy-a-[[(S)-a-isobutyl-o-piperidinobenzyl]carbamoyl]-p-toluic acid |
| MFCD00906179 |
| 2-ethoxy-4-[2-({(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl}amino)-2-oxoethyl]benzoic acid |
| Prandin,GlucoNorm,NovoNorm |
| 2-Ethoxy-4-[2-({(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl}amino)-2-oxoethyl]benzoic acid |
| Benzoic acid, 2-ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]- |
| (S)-2-Ethoxy-4-[2-[[3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoic acid |
| Repaglinide |
| 2-ethoxy-4-[2-[[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid |
| 2-ethoxy-4-(2-{[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino}-2-oxoethyl)benzoic acid |
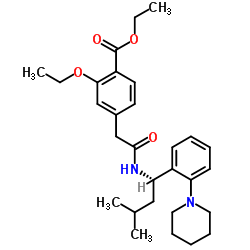 CAS#:147770-06-7
CAS#:147770-06-7 CAS#:110-89-4
CAS#:110-89-4 CAS#:72752-52-4
CAS#:72752-52-4 CAS#:873-32-5
CAS#:873-32-5 CAS#:34595-26-1
CAS#:34595-26-1
