CJ-13610
Modify Date: 2025-09-02 18:48:00
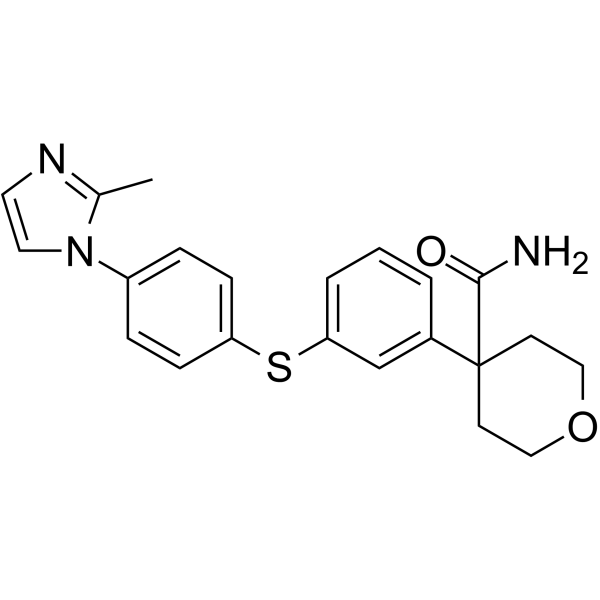
CJ-13610 structure
|
Common Name | CJ-13610 | ||
|---|---|---|---|---|
| CAS Number | 179420-17-8 | Molecular Weight | 393.50200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H23N3O2S | Melting Point | 198 - 200 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of CJ-13610CJ-13,610, a nonredox-type 5-LO inhibitor, dose dependently suppresses 5-LO product formation in ionophore A23187-stimulated PMNL in the absence of exogenous AA with an IC50 of about 70 nM[1]. PMNL: polymorphonuclear leukocytes; AA: arachidonic acid |
| Name | 4-[3-[4-(2-methylimidazol-1-yl)phenyl]sulfanylphenyl]oxane-4-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | CJ-13,610, a nonredox-type 5-LO inhibitor, dose dependently suppresses 5-LO product formation in ionophore A23187-stimulated PMNL in the absence of exogenous AA with an IC50 of about 70 nM[1]. PMNL: polymorphonuclear leukocytes; AA: arachidonic acid |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 198 - 200 °C |
|---|---|
| Molecular Formula | C22H23N3O2S |
| Molecular Weight | 393.50200 |
| Exact Mass | 393.15100 |
| PSA | 96.43000 |
| LogP | 5.01510 |
| Storage condition | 2-8°C |
| RIDADR | NONH for all modes of transport |
|---|
|
Comparative protection against liver inflammation and fibrosis by a selective cyclooxygenase-2 inhibitor and a nonredox-type 5-lipoxygenase inhibitor.
J. Pharmacol. Exp. Ther. 323(3) , 778-86, (2007) In this study, we examined the relative contribution of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO), two major proinflammatory pathways up-regulated in liver disease, to the progression of hepa... |
| Tetrahydro-4-[3-[[4-(2-methyl-1H-imidazol-1-yl)phenyl]thio]phenyl]-2H-pyran-4-carboxamide |