Thalidomide-O-amido-C4-NH2 hydrochloride
Modify Date: 2024-01-10 12:30:10
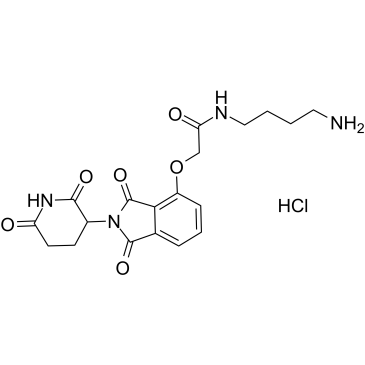
Thalidomide-O-amido-C4-NH2 hydrochloride structure
|
Common Name | Thalidomide-O-amido-C4-NH2 hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 2245697-86-1 | Molecular Weight | 438.86 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C19H23ClN4O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Thalidomide-O-amido-C4-NH2 hydrochlorideThalidomide-O-amido-C4-NH2 hydrochloride, a synthesized E3 ligase ligand-linker conjugate that incorporates the Thalidomide based cereblon ligand and a linker, can be used in the synthesis of PROTACs[1]. |
| Name | Thalidomide-O-amido-C4-NH2 hydrochloride |
|---|
| Description | Thalidomide-O-amido-C4-NH2 hydrochloride, a synthesized E3 ligase ligand-linker conjugate that incorporates the Thalidomide based cereblon ligand and a linker, can be used in the synthesis of PROTACs[1]. |
|---|---|
| Related Catalog | |
| Target |
Cereblon |
| In Vitro | Thalidomide-O-amido-C4-NH2 is an amine intermediate (Compound 41), which can be used as is a heterobifunctional PROTAC BET degrader. The bromodomain and extra-terminal (BET) family proteins, consisting of BRD2, BRD3, BRD4, and testis-specific BRDT members, are epigenetic “readers” and play a key role in the regulation of gene transcription. BET proteins are considered to be attractive therapeutic targets for cancer and other human diseases. Recently, heterobifunctional small-molecule BET degraders have been designed based upon the proteolysis targeting chimera (PROTAC) concept to induce BET protein degradation[1]. Thalidomide-O-amido-C4-NH2 is a degron-linker (refer to Compound DL6-TL). Degron-linker-targeting ligand, wherein the linker is covalently bound lo at least one degron and at least one targeting ligand, the degron is a compound capable of binding to an ubiquitin ligase such as an E3 ubiquitin ligase (e g, cereblon), and the targeting ligand is capable of binding to the targeted protein (s)[2]. |
| References |
| Molecular Formula | C19H23ClN4O6 |
|---|---|
| Molecular Weight | 438.86 |
| Hazard Codes | Xi |
|---|