Acetaminophen Acetate (Acetaminophen Impurity)

Acetaminophen Acetate (Acetaminophen Impurity) structure
|
Common Name | Acetaminophen Acetate (Acetaminophen Impurity) | ||
|---|---|---|---|---|
| CAS Number | 2623-33-8 | Molecular Weight | 193.199 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 376.4±25.0 °C at 760 mmHg | |
| Molecular Formula | C10H11NO3 | Melting Point | 155°C | |
| MSDS | Chinese USA | Flash Point | 181.4±23.2 °C | |
Use of Acetaminophen Acetate (Acetaminophen Impurity)4-Acetamidophenyl acetate is an impurity of Acetaminophen (paracetamol). Acetaminophen, an analgesic drug, is a selective COX-2 inhibitor (IC50=25.8 μM), and is a potent hepatic N-acetyltransferase 2 (NAT2) inhibitor[1][2]. |
| Name | (4-acetamidophenyl) acetate |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Acetamidophenyl acetate is an impurity of Acetaminophen (paracetamol). Acetaminophen, an analgesic drug, is a selective COX-2 inhibitor (IC50=25.8 μM), and is a potent hepatic N-acetyltransferase 2 (NAT2) inhibitor[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 376.4±25.0 °C at 760 mmHg |
| Melting Point | 155°C |
| Molecular Formula | C10H11NO3 |
| Molecular Weight | 193.199 |
| Flash Point | 181.4±23.2 °C |
| Exact Mass | 193.073898 |
| PSA | 55.40000 |
| LogP | 0.60 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.561 |
| Storage condition | Refrigerator |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | 36/37/38 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2924299090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Analysis of tablets containing aspirin, acetaminophen, and ascorbic acid by high-performance liquid chromatography.
J. Pharm. Sci. 73(12) , 1830-3, (1984) The high-performance liquid chromatographic method described enables the quantitation of the components and the main impurities of tablets containing aspirin, acetaminophen, and ascorbic acid. A C8 re... |
|
|
[N,O-diacetyl-p-aminophenol formation by combined use of aspirin with phenacetin and acetaminophen in vivo (author's transl)].
Yakugaku Zasshi 100(10) , 1043-7, (1980)
|
| 4-Acetamidophenyl acetate |
| Acetaminophen Acetate (Acetaminophen Impurity) |
| Acetaminophen acetate |
| EINECS 220-077-6 |
| 4-AcNHC6H4OAc |
| 4'-Acetoxyacetanilide |
| Diacetamat |
| P-ACETOXYACETANILIDE |
| Acetaminophen Related Compound A |
| MFCD00059205 |
| N,O-Diacetyl-4-aminophenol |
| 4-(Acetylamino)phenyl acetate,p-Acetoxyacetanilide |
| 4-Acetoxyacetanilide |
| Acetamide, N-[4-(acetyloxy)phenyl]- |
| diacetamate |
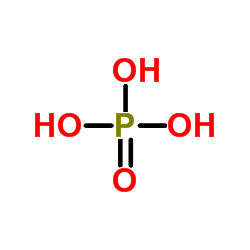 CAS#:7664-38-2
CAS#:7664-38-2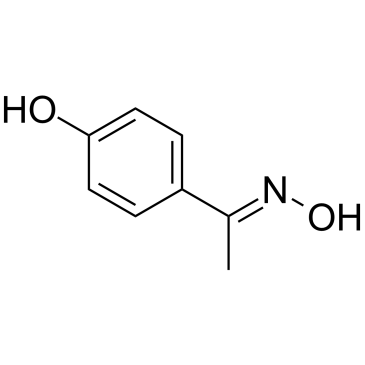 CAS#:34523-34-7
CAS#:34523-34-7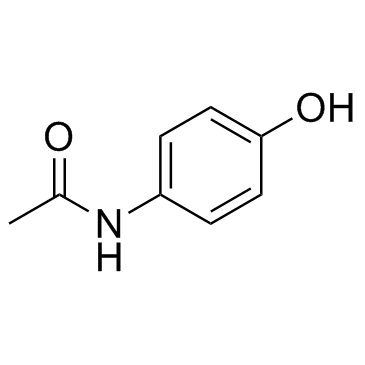 CAS#:103-90-2
CAS#:103-90-2 CAS#:75-36-5
CAS#:75-36-5![1-(2-thioxobenzo[d]oxazol-3(2H)-yl)ethanone Structure](https://image.chemsrc.com/caspic/352/37441-95-5.png) CAS#:37441-95-5
CAS#:37441-95-5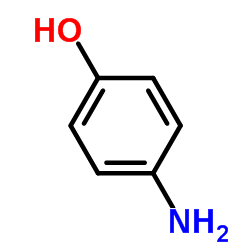 CAS#:123-30-8
CAS#:123-30-8 CAS#:108-24-7
CAS#:108-24-7 CAS#:108-95-2
CAS#:108-95-2 CAS#:64-19-7
CAS#:64-19-7 CAS#:103-84-4
CAS#:103-84-4 CAS#:95-21-6
CAS#:95-21-6 CAS#:554-84-7
CAS#:554-84-7 CAS#:26271-75-0
CAS#:26271-75-0 CAS#:13871-68-6
CAS#:13871-68-6 CAS#:62-44-2
CAS#:62-44-2 CAS#:610-81-1
CAS#:610-81-1 CAS#:7298-67-1
CAS#:7298-67-1 CAS#:141-78-6
CAS#:141-78-6