CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
WP2360000
-
CHEMICAL NAME :
-
Sulfanilamide, N(sup 1)-2-thiazolyl-
-
CAS REGISTRY NUMBER :
-
72-14-0
-
LAST UPDATED :
-
199701
-
DATA ITEMS CITED :
-
19
-
MOLECULAR FORMULA :
-
C9-H9-N3-O2-S2
-
MOLECULAR WEIGHT :
-
255.33
-
WISWESSER LINE NOTATION :
-
T5N CSJ BMSWR DZ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
250 mg/kg/23D-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - conjunctive irritation Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Skin and Appendages - dermatitis, allergic (after systemic exposure)
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1370 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
400 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1450 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
990 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Tumorigenic - tumor types after systemic administration not seen spontaneously
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2310 mg/kg/2W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Tumorigenic - tumor types after systemic administration not seen spontaneously
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Tumorigenic - tumor types after systemic administration not seen spontaneously
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
29400 mg/kg
-
SEX/DURATION :
-
male 6 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
MUTATION DATA
-
TYPE OF TEST :
-
Phage inhibition capacity
-
TEST SYSTEM :
-
Microorganism - not otherwise specified
-
DOSE/DURATION :
-
5 mg/L
-
REFERENCE :
-
JGMIAN Journal of General Microbiology. (Soc. for General Microbiology, Journal Sales, 62 London Rd., Reading, RG1 5AS, UK) V.1- 1947- Volume(issue)/page/year: 8,116,1953 *** REVIEWS *** TOXICOLOGY REVIEW JMSHAO Journal of the Mount Sinai Hospital (New York). (New York, NY) V.1-36, 1934-69. For publisher information, see MSJMAZ. Volume(issue)/page/year: 10,343,1943 *** OCCUPATIONAL EXPOSURE LIMITS *** OEL-RUSSIA:STEL 1 mg/m3 JAN 1993 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 80540 No. of Facilities: 178 (estimated) No. of Industries: 5 No. of Occupations: 9 No. of Employees: 3666 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 80540 No. of Facilities: 349 (estimated) No. of Industries: 2 No. of Occupations: 4 No. of Employees: 3171 (estimated) No. of Female Employees: 1476 (estimated)
|

 CAS#:473-42-7
CAS#:473-42-7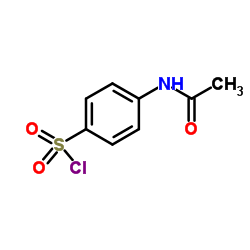 CAS#:121-60-8
CAS#:121-60-8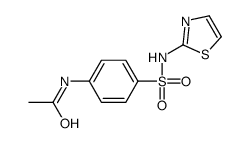 CAS#:127-76-4
CAS#:127-76-4![methyl N-[4-(1,3-thiazol-2-ylsulfamoyl)phenyl]carbamate Structure](https://image.chemsrc.com/caspic/205/33119-99-2.png) CAS#:33119-99-2
CAS#:33119-99-2 CAS#:96-50-4
CAS#:96-50-4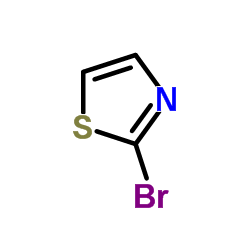 CAS#:3034-53-5
CAS#:3034-53-5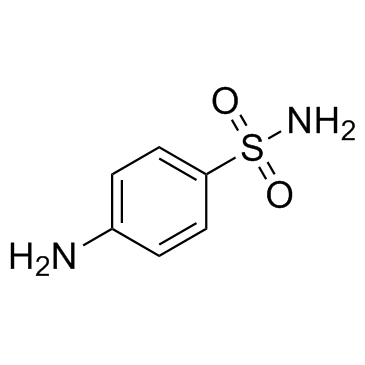 CAS#:63-74-1
CAS#:63-74-1 CAS#:5664-51-7
CAS#:5664-51-7 CAS#:24939-24-0
CAS#:24939-24-0![2-CHLORO-N-{4-[(1,3-THIAZOL-2-YLAMINO)SULFONYL]PHENYL}ACETAMIDE structure](https://image.chemsrc.com/caspic/057/104246-27-7.png) CAS#:104246-27-7
CAS#:104246-27-7![2-ethoxy-N-[4-(1,3-thiazol-2-ylsulfamoyl)phenyl]benzamide structure](https://image.chemsrc.com/caspic/400/14601-24-2.png) CAS#:14601-24-2
CAS#:14601-24-2![Benzenesulfonamide,4-[2-(6-amino-1,2,3,4-tetrahydro-2,4-dioxo-5-pyrimidinyl)diazenyl]-N-2-thiazolyl structure](https://image.chemsrc.com/caspic/180/29817-67-2.png) CAS#:29817-67-2
CAS#:29817-67-2![Benzenesulfonamide,4-[2-[2,6-diamino-5-(2-phenyldiazenyl)-3-pyridinyl]diazenyl]-N-2-thiazolyl structure](https://image.chemsrc.com/caspic/011/29817-76-3.png) CAS#:29817-76-3
CAS#:29817-76-3![2-sulfanyl-N-[4-(1,3-thiazol-2-ylsulfamoyl)phenyl]acetamide structure](https://image.chemsrc.com/caspic/113/29873-34-5.png) CAS#:29873-34-5
CAS#:29873-34-5 CAS#:85-73-4
CAS#:85-73-4![N-[4-(1,3-thiazol-2-ylsulfamoyl)phenyl]hexadecanamide structure](https://image.chemsrc.com/caspic/223/104134-71-6.png) CAS#:104134-71-6
CAS#:104134-71-6 CAS#:116-43-8
CAS#:116-43-8
