Xanthone

Xanthone structure
|
Common Name | Xanthone | ||
|---|---|---|---|---|
| CAS Number | 90-47-1 | Molecular Weight | 196.201 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 350.0±12.0 °C at 760 mmHg | |
| Molecular Formula | C13H8O2 | Melting Point | 172-174 °C(lit.) | |
| MSDS | USA | Flash Point | 169.8±13.1 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of XanthoneXanthone is isolated from Mangosteen and is known to control cell division and growth, apoptosis, inflammation, and metastasis in different stages of carcinogenesis. Xanthone has anti-oxidant, anti-tumor, anti-allergic, anti-inflammatory, anti-bacterial, anti-fungal, and anti-viral activities[1]. |
| Name | xanthone |
|---|---|
| Synonym | More Synonyms |
| Description | Xanthone is isolated from Mangosteen and is known to control cell division and growth, apoptosis, inflammation, and metastasis in different stages of carcinogenesis. Xanthone has anti-oxidant, anti-tumor, anti-allergic, anti-inflammatory, anti-bacterial, anti-fungal, and anti-viral activities[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 350.0±12.0 °C at 760 mmHg |
| Melting Point | 172-174 °C(lit.) |
| Molecular Formula | C13H8O2 |
| Molecular Weight | 196.201 |
| Flash Point | 169.8±13.1 °C |
| Exact Mass | 196.052429 |
| PSA | 30.21000 |
| LogP | 3.39 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.643 |
| InChIKey | JNELGWHKGNBSMD-UHFFFAOYSA-N |
| SMILES | O=c1c2ccccc2oc2ccccc12 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi: Irritant;Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S24/25 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | ZD5711000 |
| HS Code | 2932999099 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synergistic Effects Between Thioxanthones and Oxacillin Against Methicillin-Resistant Staphylococcus aureus.
Microb. Drug Resist. 21 , 404-15, (2015) The extensive use of antimicrobials is leaving medicine with few effective therapeutic options to treat many infections due to the fact that many organisms developed resistance to commonly used drugs.... |
|
|
Xanthones from roots, hairy roots and cell suspension cultures of selected Hypericum species and their antifungal activity against Candida albicans.
Plant Cell Rep. 34 , 1953-62, (2015) Highest xanthone contents were found in Hypericum pulchrum and H. annulatum untransformed roots. The best anti- Candida activity was obtained for hairy roots extracts of H. tetrapterum clone 2 ATCC 15... |
|
|
Identification and characterization of mefloquine efficacy against JC virus in vitro.
Antimicrob. Agents Chemother. 53 , 1840-9, (2009) Progressive multifocal leukoencephalopathy (PML) is a rare but frequently fatal disease caused by the uncontrolled replication of JC virus (JCV), a polyomavirus, in the brains of some immunocompromise... |
| Xanthone |
| Dibenzo-g-pyrone |
| Benzophenone oxide |
| 9-Xanthenone |
| MFCD00005060 |
| 9H-Xanthen-9-one |
| 9-Oxoxanthene |
| Genicide |
| Diphenylene ketone oxide |
| Xanthenone |
| EINECS 201-997-7 |
| xanthen-9-one |
| Dibenzo-γ-pyrone |
 CAS#:88284-48-4
CAS#:88284-48-4 CAS#:445-29-4
CAS#:445-29-4 CAS#:92-83-1
CAS#:92-83-1 CAS#:82-07-5
CAS#:82-07-5 CAS#:1304461-08-2
CAS#:1304461-08-2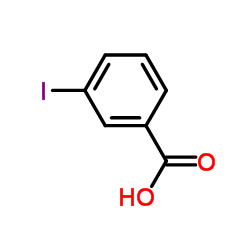 CAS#:88-67-5
CAS#:88-67-5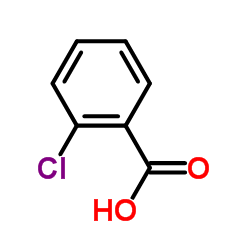 CAS#:118-91-2
CAS#:118-91-2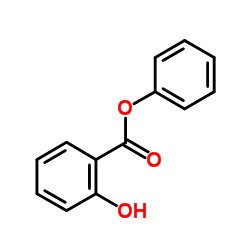 CAS#:118-55-8
CAS#:118-55-8 CAS#:76279-43-1
CAS#:76279-43-1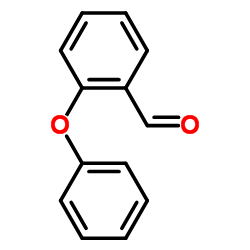 CAS#:19434-34-5
CAS#:19434-34-5 CAS#:90-46-0
CAS#:90-46-0 CAS#:117-99-7
CAS#:117-99-7 CAS#:517-45-3
CAS#:517-45-3 CAS#:4381-14-0
CAS#:4381-14-0 CAS#:27265-96-9
CAS#:27265-96-9 CAS#:632-51-9
CAS#:632-51-9 CAS#:6538-19-8
CAS#:6538-19-8 CAS#:21147-18-2
CAS#:21147-18-2 CAS#:120-51-4
CAS#:120-51-4
