Licofelone-d4
更新时间:2025-08-25 20:45:12
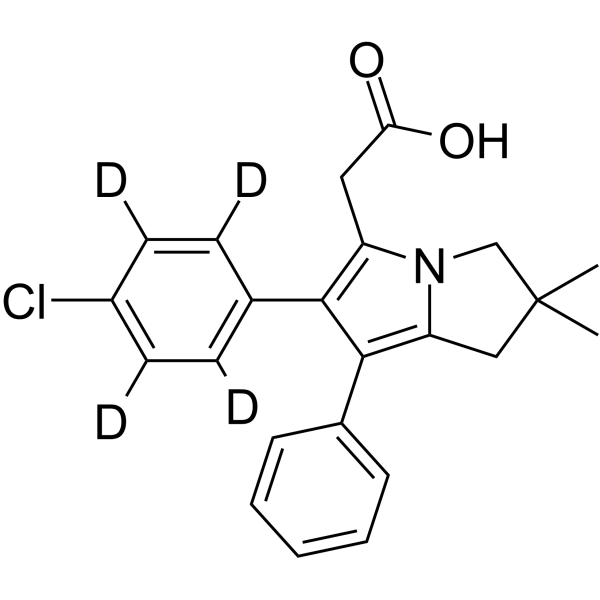
Licofelone-d4结构式

|
常用名 | Licofelone-d4 | 英文名 | Licofelone-d4 |
|---|---|---|---|---|
| CAS号 | 1189427-04-0 | 分子量 | 383.90 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C23H18D4ClNO2 | 熔点 | N/A | |
| MSDS | N/A | 闪点 | N/A |
Licofelone-d4用途利考酮-d4(ML-3000-d4)是氘标记的利考酮。利考酮(ML-3000)是一种用于治疗骨关节炎的双重COX/5-脂氧合酶(5-LOX)抑制剂(IC50分别为0.21/0.18μM)。利考酮具有抗炎和抗增殖作用。利考酮诱导细胞凋亡,并减少促炎性白三烯和前列腺素的产生[1][2][3]。 |
| 英文名 | Licofelone-d4 |
|---|
| 描述 | 利考酮-d4(ML-3000-d4)是氘标记的利考酮。利考酮(ML-3000)是一种用于治疗骨关节炎的双重COX/5-脂氧合酶(5-LOX)抑制剂(IC50分别为0.21/0.18μM)。利考酮具有抗炎和抗增殖作用。利考酮诱导细胞凋亡,并减少促炎性白三烯和前列腺素的产生[1][2][3]。 |
|---|---|
| 相关类别 | |
| 体外研究 | 氢、碳和其他元素的稳定重同位素已被纳入药物分子,主要作为药物开发过程中定量的示踪剂。氘化因其可能影响药物的药代动力学和代谢特征而受到关注[1]。 |
| 参考文献 |
| 分子式 | C23H18D4ClNO2 |
|---|---|
| 分子量 | 383.90 |
| InChIKey | UAWXGRJVZSAUSZ-OCFVFILASA-N |
| SMILES | CC1(C)Cc2c(-c3ccccc3)c(-c3ccc(Cl)cc3)c(CC(=O)O)n2C1 |

