Rp-8-CPT-cAMPS sodium
Modify Date: 2025-08-26 11:42:21
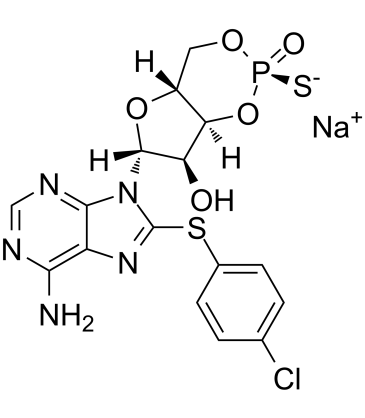
Rp-8-CPT-cAMPS sodium structure
|
Common Name | Rp-8-CPT-cAMPS sodium | ||
|---|---|---|---|---|
| CAS Number | 221905-35-7 | Molecular Weight | 509.86 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H14ClN5NaO5PS2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Rp-8-CPT-cAMPS sodiumRp-8-CPT-cAMPS sodium, a cAMP analog, is a potent and competitive antagonist of cAMP-induced activation of cAMP-dependent PKA I and II. Rp-8-CPT-cAMPS sodium preferentially selects site A of RI compares to site A of RII and site B of RII compares to site B of RI[1][2]. |
| Name | Rp-8-CPT-cAMPS sodium |
|---|
| Description | Rp-8-CPT-cAMPS sodium, a cAMP analog, is a potent and competitive antagonist of cAMP-induced activation of cAMP-dependent PKA I and II. Rp-8-CPT-cAMPS sodium preferentially selects site A of RI compares to site A of RII and site B of RII compares to site B of RI[1][2]. |
|---|---|
| Related Catalog | |
| Target |
PKA[1] |
| In Vitro | Rp-8-CPT-cAMPS (100 μM; 15 min) blocks phosphorylation of VASP by 6-Bnz-cAMP and largely reduces VASP phosphorylation by forskolin and fenoterol[2]. Rp-8-CPT-cAMPS (100 μM; 30 min) reduces GTP-loading of Rap1 by both 8-pCPT-2'-O-Me-cAMP and 6-Bnz-cAMP[2]. Rp-8-CPT-cAMPS (100 μM; 30 min) largely diminishes the augmentation of bradykinin-induced IL-8 release by the PKA activator 6-Bnz-cAMP and the Epac activator 8-pCPT-2'-O-Me-cAMP[2]. Rp-8-CPT-cAMPS (10 μM) inhibits the endothelium-dependent and -independent relaxation which induced by Venom in pre-contracted rat mesenteric artery rings[3]. |
| References |
| Molecular Formula | C16H14ClN5NaO5PS2 |
|---|---|
| Molecular Weight | 509.86 |