Acacetin
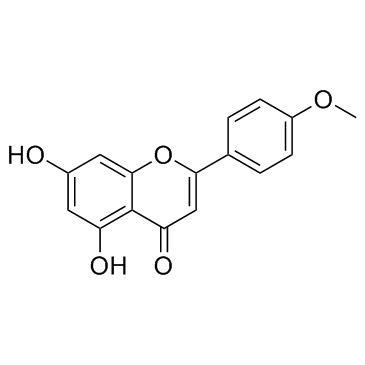
Acacetin structure
|
Common Name | Acacetin | ||
|---|---|---|---|---|
| CAS Number | 480-44-4 | Molecular Weight | 284.263 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 518.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H12O5 | Melting Point | 260-265 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 198.3±23.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Acacetin1) Natural acacetin was a 4.0-fold and 5.5-fold more potent inhibitor of BACE-1 than oleanolic acid and maslinic acid, respectively.[1]2) Acacetin significantly suppressed the photoreceptor collapse. [1]3) Acacetin significantly reduces the Aβ levels by interfering with human APP proteolytic processing and BACE-1 expression. [1]4) Acacetin inhibited the generation of the APP-CTF by affecting APP cleavage. [1]5) Acacetin prolongs lifespan of significantly in the dose dependent manner. Acacetin(25 uM) had the greatest effect on longevity, extending mean lifespan significantly by 27.31% at 25 uM concentration |
| Name | 5,7-dihydroxy-4'-methoxyflavone |
|---|---|
| Synonym | More Synonyms |
| Description | 1) Natural acacetin was a 4.0-fold and 5.5-fold more potent inhibitor of BACE-1 than oleanolic acid and maslinic acid, respectively.[1]2) Acacetin significantly suppressed the photoreceptor collapse. [1]3) Acacetin significantly reduces the Aβ levels by interfering with human APP proteolytic processing and BACE-1 expression. [1]4) Acacetin inhibited the generation of the APP-CTF by affecting APP cleavage. [1]5) Acacetin prolongs lifespan of significantly in the dose dependent manner. Acacetin(25 uM) had the greatest effect on longevity, extending mean lifespan significantly by 27.31% at 25 uM concentration |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 518.6±50.0 °C at 760 mmHg |
| Melting Point | 260-265 °C(lit.) |
| Molecular Formula | C16H12O5 |
| Molecular Weight | 284.263 |
| Flash Point | 198.3±23.6 °C |
| Exact Mass | 284.068481 |
| PSA | 79.90000 |
| LogP | 3.15 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.669 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DJ3002000 |
| HS Code | 2914509090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Five hTRPA1 Agonists Found in Indigenous Korean Mint, Agastache rugosa.
PLoS ONE 10 , e0127060, (2015) Transient receptor potential ankyrin1 (TRPA1) and transient receptor potential vanilloid 1 (TRPV1) are members of the TRP superfamily of structurally related, nonselective cation channels and mediator... |
|
|
Flavonoids inhibit COX-1 and COX-2 enzymes and cytokine/chemokine production in human whole blood.
Inflammation 38(2) , 858-70, (2015) Cyclooxygenase 2 (COX-2) and the production of cytokines/chemokines are important targets for the modulation of the inflammatory response. Although a large variety of inhibitors of these pathways have... |
|
|
Simultaneous quantification of 25 active constituents in the total flavonoids extract from Herba Desmodii Styracifolii by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry.
J. Sep. Sci. 38(7) , 1156-63, (2015) A sensitive and selective high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry method has been developed and validated for the simultaneous determinatio... |
| Linarigenin |
| 5,7-Dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one |
| 5,7-Dihydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one |
| Acacetin |
| 5,7-dihydroxy-2-(4-methoxyphenyl)chromen-4-one |
| 5,7-Dihydroxy-4'-methoxyflavone |
| Apigenin-4'-methyl Ether |
| 4'-O-Methylapigenin |
| Buddleoflavonol |
| MFCD00016936 |
| 4'-Methoxyapigenin |
| 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-methoxyphenyl)- |
| Apigenin 4'-methyl ether |
| EINECS 207-552-3 |
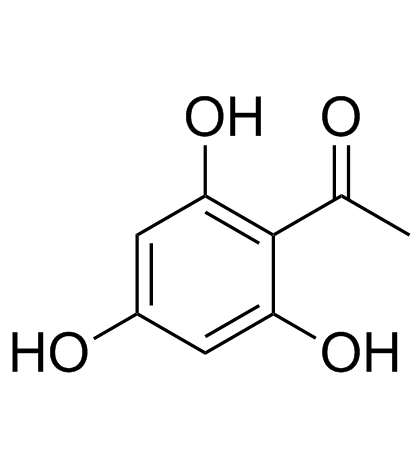 CAS#:480-66-0
CAS#:480-66-0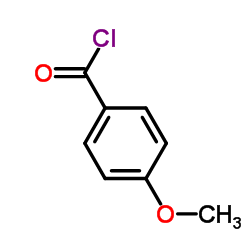 CAS#:100-07-2
CAS#:100-07-2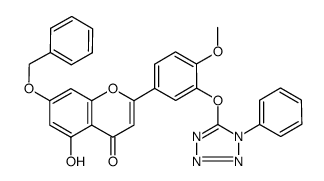 CAS#:771481-08-4
CAS#:771481-08-4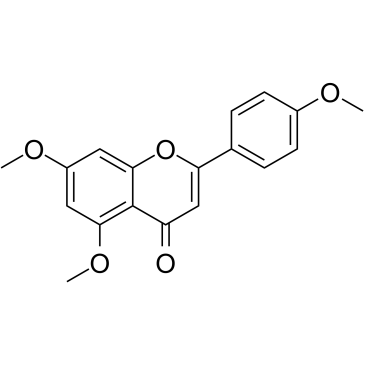 CAS#:5631-70-9
CAS#:5631-70-9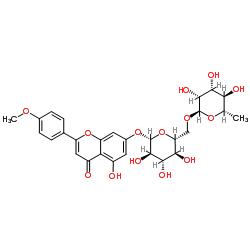 CAS#:480-36-4
CAS#:480-36-4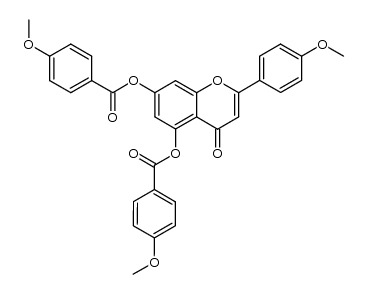 CAS#:110865-07-1
CAS#:110865-07-1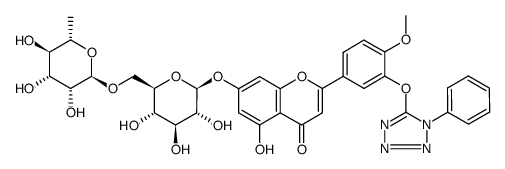 CAS#:771480-96-7
CAS#:771480-96-7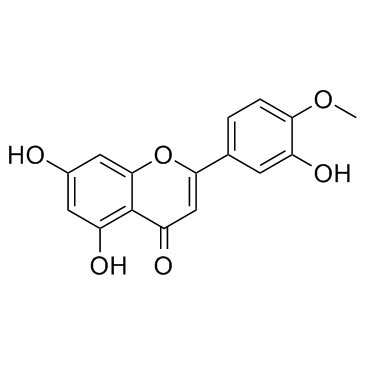 CAS#:520-34-3
CAS#:520-34-3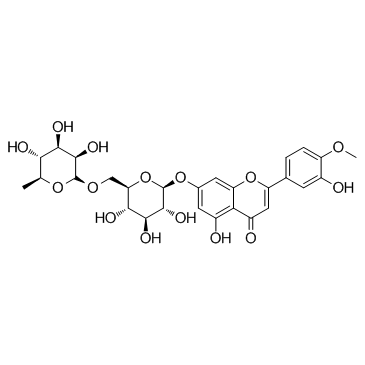 CAS#:520-27-4
CAS#:520-27-4 CAS#:108-73-6
CAS#:108-73-6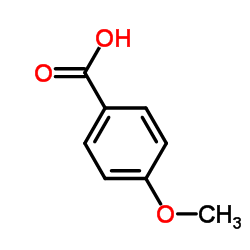 CAS#:100-09-4
CAS#:100-09-4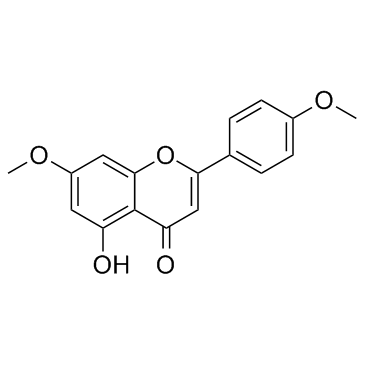 CAS#:5128-44-9
CAS#:5128-44-9 CAS#:480-43-3
CAS#:480-43-3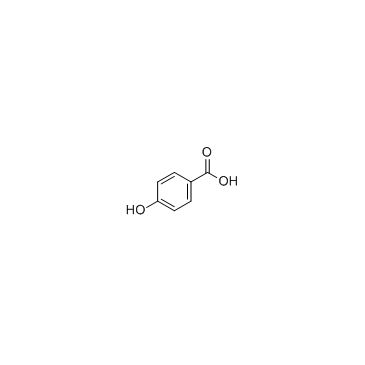 CAS#:99-96-7
CAS#:99-96-7 CAS#:529-53-3
CAS#:529-53-3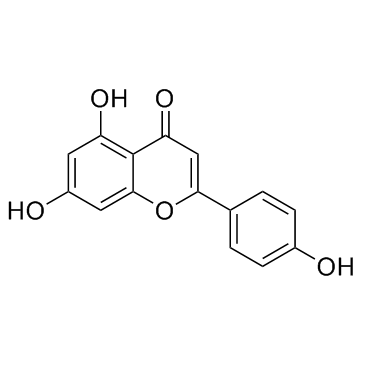 CAS#:520-36-5
CAS#:520-36-5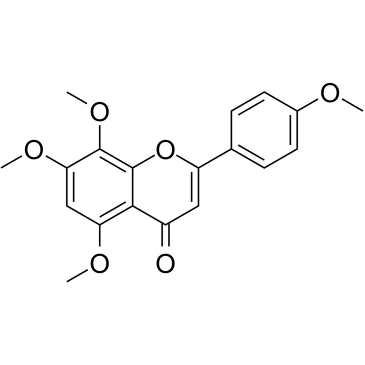 CAS#:6601-66-7
CAS#:6601-66-7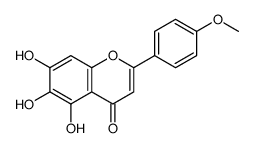 CAS#:6563-66-2
CAS#:6563-66-2
