Ubiquinone Q0
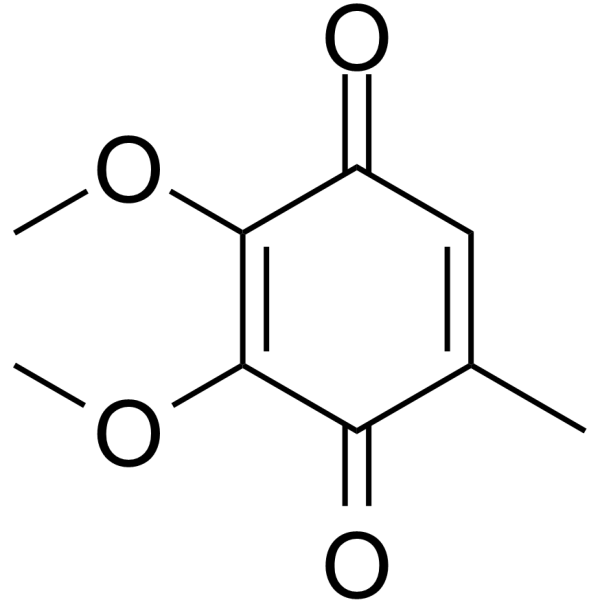
Ubiquinone Q0 structure
|
Common Name | Ubiquinone Q0 | ||
|---|---|---|---|---|
| CAS Number | 605-94-7 | Molecular Weight | 182.173 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 331.4±42.0 °C at 760 mmHg | |
| Molecular Formula | C9H10O4 | Melting Point | 58-60 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 148.6±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Ubiquinone Q0Coenzyme Q0 (CoQ0) is a potent, oral active ubiquinone compound can be derived from Antrodia cinnamomea. Coenzyme Q0 induces apoptosis and autophagy, suppresses of HER-2/AKT/mTOR signaling to potentiate the apoptosis and autophagy mechanisms. Coenzyme Q0 regulates NFκB/AP-1 activation and enhances Nrf2 stabilization in attenuation of inflammation and redox imbalance. Coenzyme Q0 has anti-angiogenic activity through downregulation of MMP-9/NF-κB and upregulation of HO-1 signaling[1][2][3]. |
| Name | ubiquinone-0 |
|---|---|
| Synonym | More Synonyms |
| Description | Coenzyme Q0 (CoQ0) is a potent, oral active ubiquinone compound can be derived from Antrodia cinnamomea. Coenzyme Q0 induces apoptosis and autophagy, suppresses of HER-2/AKT/mTOR signaling to potentiate the apoptosis and autophagy mechanisms. Coenzyme Q0 regulates NFκB/AP-1 activation and enhances Nrf2 stabilization in attenuation of inflammation and redox imbalance. Coenzyme Q0 has anti-angiogenic activity through downregulation of MMP-9/NF-κB and upregulation of HO-1 signaling[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Coenzyme Q0 (0-40 µM; 24 h) and inhibits viability and growth of human ovarian carcinoma cells[1]. Coenzyme Q0 (CoQ0) (0-30 µM; 24 h; SKOV-3 cells) has anti-proliferative activity through induction of G2/M cell-cycle arrest and reduction of cell-cycle regulatory proteins[1]. Coenzyme Q0 (CoQ0) (0-30 µM; 0-30 min; SKOV-3 cells) increases intracellular ROS levels to promote SKOV-3 cell death[1]. Coenzyme Q0 (CoQ0) (0-30 µM; 24 h; SKOV-3 cells) induces autophagy by increase accumulation of LC3-II, GFP-LC3 puncta, AVOs formation and Beclin-1/Bcl-2 dysregulation[1]. Coenzyme Q0 (CoQ0) (0-30 µM; 24 h; SKOV-3 cells) induces apoptosis by mitochondrial (caspase-3, PARP and Bax/Bcl-2 dysregulation) and ER stress (caspase-12 and Hsp70) signals[1]. Coenzyme Q0 (CoQ0) (30 µM; 24 h; SKOV-3 cells) suppresses of HER-2/AKT/mTOR signaling to potentiate the apoptosis and autophagy mechanisms[1]. Coenzyme Q0 (CoQ0) (0-10 µM; 0.5-18 h; RAW264.7 cells) regulates NFκB/AP-1 activation and enhances Nrf2 stabilization[2]. Coenzyme Q0 (CoQ0) (5 µM; 0-12 h; EA.hy 926 cells) has anti-angiogenic activity in EA.hy 926 cells[3]. Cell Viability Assay[1] Cell Line: SKOV-3, A2780 and A2870/CP70 cells Concentration: 0, 10, 20, 30 and 40 µM Incubation Time: 24 hours Result: Decreased viability with the IC50 values of 26.6 µM, 27.3 µM and 28.4 µM for SKOV-3, A2780 and A2870/CP70 cells, respectively. Cell Cycle Analysis[1] Cell Line: SKOV-3, A2780 and A2870/CP70 cells Concentration: 0, 10, 20 and 30 µM Incubation Time: 24 hours Result: Arrested cell cycle at G2/M phase and reduced cell-cycle proteins in SKOV-3 cells. Apoptosis Analysis[1] Cell Line: SKOV-3, A2780 and A2870/CP70 cells Concentration: 0, 5, 15 and 30 µM Incubation Time: 24 hours Result: Promoted the conversion of LC3–1 to LC3-II and increased the LC3-II accumulation. Increased Bax/Bcl-2 ratio in a dose-dependent manner. Apoptosis Analysis[1] Cell Line: SKOV-3 cells Concentration: 0, 10, 20 and 30 µM Incubation Time: 24 hours Result: Had the percentage of early apoptotic cells are 25.1%, 34% and 36% for 10, 20 and 30 µM, respectively. Western Blot Analysis[1] Cell Line: SKOV-3 cells Concentration: 0, 5, 15 and 30 µM Incubation Time: 24 hours Result: Activated of caspase-3 and cleavaged of PARP. Increased the expressions of caspase-12, HSP-70 and Bax in a dose-dependent manner, decreased the expressions of Bcl-2. Western Blot Analysis[1] Cell Line: SKOV-3 cells Concentration: 30 µM Incubation Time: 24 hours Result: Decreased the phosphorylated HER-2 (Y1221) levels, p-AKT (Ser473) and p-mTOR (S2448) levels. Western Blot Analysis[2] Cell Line: RAW264.7 cells Concentration: 0, 2.5, 5 and 10 µM Incubation Time: 0.5-18 hours Result: Inhibited iNOS/COX-2 protein expressions with reductions of NO, PGE2, TNF-α and IL-1β secretions. Western Blot Analysis[3] Cell Line: EA.hy 926 cells Concentration: 5 µM Incubation Time: 0, 1, 3, 6 and 12 hours Result: Increased expressions of heme oxygenase-1 (HO-1) and γ-glutamylcysteine synthetase (γ-GCLC), inhibits protein expressions of matrix metalloproteinase-9 (MMP-9), reduces TNF-α-induced nuclear translocation and transcriptional activation of nuclear factor-κB (NF-κB). |
| In Vivo | Coenzyme Q0 (CoQ0) (1.5 and 2.5 mg/kg; i.p.; once every four days, for 52 d) suppresses tumor growth in SKOV-3 xenografted nude mice[1]. Coenzyme Q0(CoQ0) (5 mg/kg; p.o.; for 4 h) has anti-inflammatory activities through Nrf2 activation and NFκB inhibition in liver and spleen of LPS-treated mice[2]. Animal Model: SKOV-3 xenografted nude mice[1] Dosage: 1.5 and 2.5 mg/kg Administration: Intraperitoneal injection; Once every four days, for 52 days Result: Inhibited the tumor growth at 1.5 and 2.5 mg/kg. Animal Model: LPS-treated female FVB mice[2] Dosage: 5 mg/kg Administration: Oral administration; for 4 hours Result: Down-regulates inflammatory genes in liver and spleen tissues of LPS injected mice. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 331.4±42.0 °C at 760 mmHg |
| Melting Point | 58-60 °C(lit.) |
| Molecular Formula | C9H10O4 |
| Molecular Weight | 182.173 |
| Flash Point | 148.6±27.9 °C |
| Exact Mass | 182.057907 |
| PSA | 52.60000 |
| LogP | 0.12 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.498 |
| InChIKey | UIXPTCZPFCVOQF-UHFFFAOYSA-N |
| SMILES | COC1=C(OC)C(=O)C(C)=CC1=O |
| Storage condition | 2-8°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, reducing agents. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
|
Cofactor Specificity of the Bifunctional Alcohol and Aldehyde Dehydrogenase (AdhE) in Wild-Type and Mutant Clostridium thermocellum and Thermoanaerobacterium saccharolyticum.
J. Bacteriol. 197 , 2610-9, (2015) Clostridium thermocellum and Thermoanaerobacterium saccharolyticum are thermophilic bacteria that have been engineered to produce ethanol from the cellulose and hemicellulose fractions of biomass, res... |
|
|
Prerequisites for ubiquinone analogs to prevent mitochondrial permeability transition-induced cell death.
J. Bioenerg. Biomembr. 44(1) , 207-12, (2012) The permeability transition pore (PTP) is a mitochondrial inner membrane channel involved in cell death. The inhibition of PTP opening has been proved to be an effective strategy to prevent cell death... |
|
|
Ubiquinone analogs: a mitochondrial permeability transition pore-dependent pathway to selective cell death.
PLoS ONE 5 , e11792, (2010) Prolonged opening of the mitochondrial permeability transition pore (PTP) leads to cell death. Various ubiquinone analogs have been shown to regulate PTP opening but the outcome of PTP regulation by u... |
| 1,2,3-Dimethoxy-5-methyl-1,4-benzoquinone |
| 2,5-Cyclohexadiene-1,4-dione, 2,3-dimethoxy-5-methyl- |
| EINECS 210-100-8 |
| 2,3-dimethoxy-5-methyl-1,4-benzoquinone |
| 2,3-Dimethoxy-5-methylcyclohexa-2,5-dien-1,4-dion |
| p-Benzoquinone, 2,3-dimethoxy-5-methyl- |
| 2,3-Dimethoxy-5-Methyl-p-Benzoquinone |
| Ubiquinone Q0 |
| 2,3-dimethoxy-5-methylcyclohexa-2,5-diene-1,4-dione |
| MFCD00001595 |
 CAS#:6443-69-2
CAS#:6443-69-2 CAS#:1128-32-1
CAS#:1128-32-1 CAS#:39068-88-7
CAS#:39068-88-7 CAS#:494-99-5
CAS#:494-99-5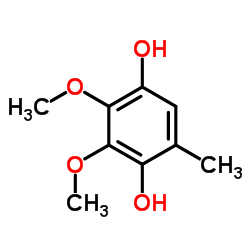 CAS#:3066-90-8
CAS#:3066-90-8 CAS#:35896-58-3
CAS#:35896-58-3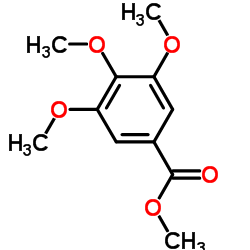 CAS#:1916-07-0
CAS#:1916-07-0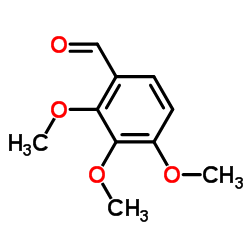 CAS#:2103-57-3
CAS#:2103-57-3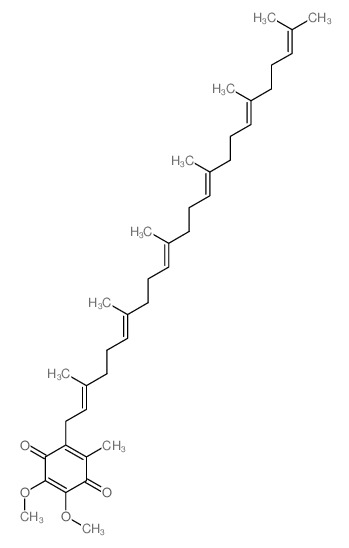 CAS#:1065-31-2
CAS#:1065-31-2 CAS#:490-83-5
CAS#:490-83-5 CAS#:303-95-7
CAS#:303-95-7 CAS#:303-98-0
CAS#:303-98-0 CAS#:73875-27-1
CAS#:73875-27-1 CAS#:132080-64-9
CAS#:132080-64-9 CAS#:34417-79-3
CAS#:34417-79-3 CAS#:303-97-9
CAS#:303-97-9
